Chronic Exposure to Free Fatty Acid Reduces Pancreatic β-Cell Insulin Content by Increasing Basal Insulin Secretion That Is Not Compensated For by a Corresponding Increase in Proinsulin Biosynthesis Translation
Coming next in the discussion of diabetes I am going to discuss insulin secretion in greater detail. I was reminded of this post in the writing process. So ... Bump!
Original Publish Date: 4/4/11
Chronic Exposure to Free Fatty Acid Reduces Pancreatic β-Cell Insulin Content by Increasing Basal Insulin Secretion That Is Not Compensated For by a Corresponding Increase in Proinsulin Biosynthesis Translation
JD McGarry contributing author.
Original Publish Date: 4/4/11
Chronic Exposure to Free Fatty Acid Reduces Pancreatic β-Cell Insulin Content by Increasing Basal Insulin Secretion That Is Not Compensated For by a Corresponding Increase in Proinsulin Biosynthesis Translation
JD McGarry contributing author.
{Please note: Excerpts from the text will be edited somewhat to avoid "cluttering" references, statistical values, and some rounding of numbers. Text will sometimes be presented in bullet form or with paragraph breaks to ease reading. It is not my intent to plagiarize nor to alter the content. If anyone feels I've altered the content in any meaningful way, do please let me know!} Direct quotes will be indented.
FFA are an important physiological fuel for islets, and act as a supplemental nutrient secretagogue to potentiate insulin release acutely in the presence of glucose.
Moreover, there is emerging evidence that transient elevation of cytosolic longchain fatty acyl-CoA as a consequence of increased glycolytic flux in the β-cell is a critical step for metabolic coupling of glucose-stimulated insulin release. However, chronically elevated FFA are believed to play a role in the pathogenesis of certain forms of type II diabetes by both inhibiting insulin stimulated peripheral glucose uptake and contributing to β-cell dysfunction. In contrast to short-term effects of FFA, prolonged exposure to FFA has detrimental effects for β-cell function, including impairment of glucose-induced insulin release as well as other metabolic and morphological abnormalities.
Again, the concerning thing to me here is that it's the exposure of the β-cell to FFA's - a direct effect - that is the problem. FFA's can be elevated beyond usual levels on VLC diets. Can the β-cell tell the difference if there are not "glucose spikes"?
Under normal physiological circumstances, the pancreatic β-cell maintains a remarkably stable balance between insulin secretion and insulin production. Whenever glucose stimulates insulin release, there is a rapid and corresponding glucose-induced increase in proinsulin biosynthesis at the translational level that efficiently replenishes intracellular insulin stores. A similar scenario applies to the vast majority of nutrient secretagogues of the β-cell.
Both glucose and other peptides (like intestinal incretins) stimulate insulin secretion and an immediate re-stocking of the stores for the next time it's needed.
Although short-term exposure to FFA markedly potentiates glucose-induced insulin release, the effect of FFA on proinsulin biosynthesis remains relatively undefined. Indeed, recent studies have indicated that in contrast to FFA potentiating glucose-induced insulin secretion, FFA inhibit glucose-induced proinsulin biosynthesis. It follows that, unlike the stimulus–response coupling pathway for glucose-induced insulin release where FFA play an important signaling role, these lipid moieties are unlikely to be involved in the metabolic signal transduction pathway for proinsulin biosynthesis at the translational level.
So glucose induces insulin secretion and proinsulin formation to replace that used, while FFA magnify insulin secretion they don't cause proinsulin formation or may even suppress it.
If this is so, the insulin content of the β-cell cannot be rapidly replenished after acute stimulation of insulin release by FFA. Under normal circumstances, only a small proportion of the β-cell’s insulin intracellular store is released after an acute stimulation by a secretagogue, so that short-term FFA-induced insulin release would have little adverse effect on the β-cell’s secretory capacity. However, chronic exposure to FFA could severely deplete the internal insulin stores since there is apparently no biosynthetic backup to compensate for FFA-induced insulin hypersecretion. This study addresses the question as to how maintenance of β-cell insulin stores is affected by chronic elevated level of FFA.
I've tried to summarize the experiments below. If it gets too tedious (it was for me!), you might just want to skip to the Discussion section :-)
Part I: In vivo lipid infusions (lard oil + heparin = free fatty acids) in normal rats (300g male Sprague Dawley). Blood glucose was maintained at 120-130 mg/dL.
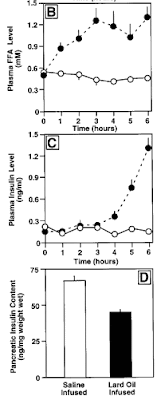
The results for plasma FFA, insulin and pancreatic insulin are shown below (left off glucose as it was just held constant). Solid dots are for FFA infusion, open circles for control infusion.
We see that in the first three hours the FFA infusion resulted in a progressive rise of FFA levels to approximately double baseline values. This was not accompanied by a change in circulating insulin. (We don't know pancreatic insulin as that could only be determined by sacrificing the animals). But with continued elevation of the fatty acids, starting around an hour after FFA's doubled, plasma insulin rose substantially.
Fatty acid infusion of normoglycemic rats increases insulin secretion and lowers pancreatic insulin content in vivo. ... total plasma FFA levels increased two to threefold at 1–6 h in lard oil–infused rats compared with saline-infused controls.
- Under the continuous normoglycemic conditions applied, it was found that after 4 h of lard oil infusion there was a twofold increase in circulating insulin levels over saline infused control rats that increased to fourfold at 5 h and to eightfold by 6 h. {basically doubling each hour}
I do think it's worth noting that the BG was maintained a bit high for rats. How much this affected the insulin depletion is not clear. But likewise, less insulin is secreted in response to glucose infusions (which is what glycemic "clamps" are) compared to oral delivery of glucose.
- After 6 h of lard oil infusion, it was found that there was a decrease (29.9%) in pancreatic insulin content compared with saline-infused control animal.
Part II: In vitro incubations of β-cells isolated from the same type of rat with oleate (a common long chain MUFA). Glucose in medium was 5.6 mM. Incubation time = 48 hours.
Islets incubated in the presence of oleate released 4.5-fold more insulin than did control islets from 6 h onwards. While in control islets, the intracellular insulin content remained constant throughout the 48-h incubation period; in those exposed to oleate it gradually decreased with time to 47% that of the control value by 24 h, and remained at this reduced level for the subsequent 24-h period.
After a 6-h exposure to oleate in vitro, insulin secretion was increased threefold, and the islet insulin content fell by 25% compared with the control. This in vitro decrease in β-cell insulin content was compared with {sic, I think they meant "comparable to" rather than "compared with"} that seen in vivo with lard oil infusion.
Part III: In vitro incubation for 3, 6, 12, 24, or 48 h at 5.6 mM glucose, 125 μM oleate, with subsequent glucose challenge with a basal (2.8 mM) or a stimulatory (16.7 mM) concentration of glucose for 1h. Insulin secretion, islet insulin content, and proinsulin biosynthesis were determined.
Part IV: In vitro culture of islets with 5.6 mM glucose and with or without 125 μM oleate for 0, 3, 12, or 24 hours. Preproinsulin mRNA, actin mRNA and 28S-rRNA were measured. Direct quote:
Direct quote:
- There was a 2-4 fold increase in basal insulin in the presence of oleate
- Glucose stimulated insulin secretion was significantly blunted.
- Islet insulin content was not altered in response to glucose with or w/o oleate, but
- Islet insulin content was diminished in oleate treated cells
- Proinsulin biosynthesis diminished with time of incubation with oleate
...the inhibitory effect of oleate on glucose-induced proinsulin biosynthesis was in contrast to the potentiating effects on insulin secretion seen in the very same islets
Part IV: In vitro culture of islets with 5.6 mM glucose and with or without 125 μM oleate for 0, 3, 12, or 24 hours. Preproinsulin mRNA, actin mRNA and 28S-rRNA were measured. Direct quote:
...control β-cells [w/o oleate] cultured in vitro at 5.6 mM glucose showed a decline in total preproinsulin mRNA content, while levels of actin mRNA remained stable. However, islets cultured in the presence of oleate maintained their preproinsulin mRNA levels, resulting in a two- to threefold increase above that in the control islets. The specific nature of oleate stimulation of preproinsulin mRNA levels was indicated, in that actin mRNA levels were unaffected by exposure to the fatty acid.Part V: Somatostatin-mediated inhibition of oleate-induced increases in basal insulin secretion. (IOW, artificially prevent insulin secretion) maintains intracellular insulin content.
Discussion:
- Elevated levels of long-chain FFA are potent stimulators of insulin secretion
- Hypothesis: transient elevation of LCFA's in β-cells, as a consequence of either increased glycolytic metabolism and/or an acute increase in exogenous FFA concentration, is important as a metabolic signaling system triggering insulin secretion.
- FFA's are "required" for insulin secretion but prolonged elevated levels can impair β-cell function.
Direct Quote: Given the role that fatty acid moieties might play in triggering insulin release, such chronic exposure to FFA could evoke a continual stimulation of insulin release with subsequent hyperinsulinemia that is commonly observed in obesity and NIDDM. Indeed, persistent elevated circulating FFA levels have been proposed as a major pathogenic factor for obesity and NIDDM since they can influence both insulin sensitivity and β-cell dysfunction .What this study demonstrated in many ways, both in vivo and in isolated β-cells was that increasing FFA levels under normal glycemic conditions increased insulin production while decreasing β-cell insulin content. Oleate, however, "specifically inhibited glucose-regulated proinsulin biosynthesis at the translational level." Therefore the FFA's cause a decrease in cellular insulin content due to a failure of fatty acids to stimulate proinsulin biosynthesis.
Direct quote: "... it should also be considered that FFA-mediated elevation of basal insulin secretion resulted in a significant reduction in the insulin content of pancreatic islet β-cells. Thus, when insulin secretion was considered as a percentage of the intracellular insulin content, it was found that FFA significantly potentiated glucose-induced insulin release as well as elevating basal insulin secretion. Viewed in this way, chronic exposure of pancreatic islets to FFA over a 48-h period in vitro enhanced rather than inhibited glucose-induced insulin release."
... there [is] an apparent adaptation of the β-cell to maintain its intracellular insulin content, albeit at a reduced level, to compensate partly for hypersecretion of insulin after 24 h of exposure to oleate. One possible explanation for this observation could be the difference in threshold glucose concentration needed to stimulate proinsulin biosynthesis vs. insulin secretion.If I'm interpreting this correctly, elevated FFA's stimulate chronic/basal insulin secretion but not the pathways to replenish the β-cells' insulin supply. When stimulated with glucose, the insulin response is excessive but eventually the β-cells adapt and shuttle resources to make more insulin in the cells rather than secreting it. In diabetics this is what is manifested by an elevated basal insulin but impaired postprandial insulin response.
Yes: "... further experiments will be required to define better the mechanism through which a reduced steady-state islet insulin content is sustained in the face of FFA-induced insulin hypersecretion."
In general, nutrients that stimulate insulin release correspondingly increase proinsulin biosynthesis to effect a balance between (pro)insulin secretion and biosynthesis that maintains β-cell insulin stores at an optimal level. However, FFA appear to be an exception to this rule. While long-chain FFA moieties are involved in the metabolic stimulus–response coupling pathway for nutrient-stimulated insulin release, they are apparently not required for the metabolic signal transduction pathway for nutrient-induced stimulation of proinsulin biosynthesis at the translational level.Given that FFA are normally elevated in the fasted state, this makes sense to me. The FFA's are primarily responsible for the feedback loop regulating their release from adipose tissue. In the immediate term, the pancreas is more interested in performing this function than anticipating a glucose challenge. Seems about right.
I'm just going to directly quote from here:
Regardless, it should be noted that in spite of FFA-induced upregulation of preproinsulin mRNA levels, there was no corresponding effect on proinsulin biosynthesis at the translational level. There are essentially two pools of preproinsulin mRNA in β-cells: one in a ribosome-free storage compartment in the cytosol, and the other with ribosomes attached, actively undergoing proinsulin biosynthesis translation located mainly on the rough endoplasmic reticulum. It follows that FFA-induced increases in preproinsulin mRNA levels were likely contributing to a quiescent cytoplasmic storage pool. Such findings emphasize the importance of translational regulation of proinsulin biosynthesis in maintenance of β-cell insulin content.
In obesity, insulin resistance, hyperinsulinemia, and hyperlipacidemia coexist, and may contribute to a clinical state of non-insulin-dependent diabetes mellitus. However, in humans, although there is a degree of correlation between obesity and type II diabetes, a good proportion of hyperlipacidemic obese individuals do not present symptoms of diabetes. The current work shows that while intracellular insulin stores decreased by 50% with 24h exposure to FFA, the ability of the β-cell to generate an adequate output of insulin in the presence of FFA was not lost relative to control islets. While this result does not rule out a reduction in insulin secretory capacity of the β-cell with a more prolonged exposure to FFA, it is probable that other factors in addition to hyperlipidemia contribute to the pathogenesis of NIDDM. It is well-established that hyperglycemia can also lead to severe β-cell dysfunction. Therefore, a combination of chronic hyperlipidemia and hyperglycemia more likely leads to reduced insulin secretory capacity, β-cell exhaustion, and onset of diabetes, which would only be worsened by the additional presence of insulin resistance.I would say the jury is still out and worthy of consideration whether elevated FFA over prolonged periods w/o hyperglycemia (e.g. VLC diet) might lead to permanent changes in β-cell function. Postprandial insulin release has the purpose, in normal individuals, of suppressing NEFA release from adipose tissue. To the degree that fats indirectly stimulate an acute insulin response (by stimulating incretins that signal insulin secretion) such a purpose would be to assure proper "trapping" of FFA in the postprandial state.
We certainly don't know if the chronically elevated FFA is benign, that's for sure. The "we're burning fatty acids at a higher rate" explanation is insufficient for me with respect to insulin's various other functions in the body other than glucose transport. I would note that this group seems to believe the "exhausted pancreas" theory, but proposes a scenario consistent with Frayn's description of the progression of IR. So: Larger adipocytes lose their sensitivity to insulin. With HSL suppression impaired, fat cells release excessive free fatty acids and/or are less adept at trapping those released by LPL in the postprandial state. Circulating NEFA levels rise. Exposure of the pancreas to prolonged periods of elevated NEFA leads to basal hyperinsulinemia and initially postprandial hyperinsulinemia. This may progress in some to where the β-cell loses its ability to mount an appropriate acute insulin response when called upon. That is β-cell dysfunction.
Can we "exhaust" the pancreas in the absence of hyperglycemia? What of the idea that metabolically the body is in the fasted state even when being fed when it is not given dietary carbohydrate. Is this optimal? Is this healthy?
In light of various recent controversies, I think it is important that we actually look at the science, and especially when diet book authors are pointing to it in support of their dietary prescriptions and proscriptions. There are few if any long term weight stable studies looking at the VLCVHF diets many advocate. Which leaves us to a handful of cultures that may have eaten a higher fat diet from wild animals as examples to speculatively extrapolate diets composed of pastured animals and cultivated vegetables. Further, as I have pointed out here before, those studies that do exist bearing the name of a diet -- be it low carb or paleo -- are not so low carb or fatty red meat-centric after all.

Comments
Paleo as practiced by some and traditional Neo are not so much worlds apart. At least that's what I get from Stephan Guyenet for one.
I think for me this is the most scary side effect of VLC diet. This is especially true since I'm at risk for NIDDM. I know there is the immediate effect of apparent blood sugar control, but it seems to be at the expense of beta cell function.
Thanks for posting this. I'm still sort of confused about what I should be eating, but I think VLC is not it.
(1) It's done in rats and in rat beta cells, not in humans.
(2) In the in vivo experiments, fatty acids were infused into a vein, not into the stomach or duodenum, where pancreatic responses would more closely follow a physiologic pattern.
(3) The fatty acids were infused for six hours, nothing comparable to a meal-like bolus.
(4) Blood glucose was held at 120-130 mg/dl in the rats. Wouldn't we expect that the pancreas would fight to bring down that glucose level during the duration of the experiment? Aren't we looking at elevated FFA's in the context of high blood glucose rather that at FFA's by themselves?
Using a fatty acid infusion strangely mimics the FFA levels seen in people on a low carb/high fat diet as you can see here in this post: http://carbsanity.blogspot.com/2011/02/failure-of-lchf-diets-to-suppress-nefa.html
I agree the bg level was kept way too high. In the above study it was ~85 mg/dl, and this would have been more appropriate. I think the main problem with VLC is that the FFA levels are high all the time and the body never gets a break.
Sorry for the typo in your name.
Do you have a reference that shows that high FFA (over several months, not in a few hours or days) are damaging in the context of constant low insulin and low blood sugar?
(1) Although ideally I suppose I could confine myself to only addressing human studies, I see no reason to dismiss a study solely on the basis of it being in an animal model. Since they had to sacrifice the animals to obtain certain data, this couldn't be done in humans, and the researchers used rat cells for the in vitro components to be consistent with their in vivo model. Does a human pancreas function differently from a rat pancreas?
(2) I disagree. Dietary fat is packaged up as chylomicrons. Circulating FFA are liberated by LPL in the bloodstream or by HSL from adipose tissue and delivered to circulation. In the fasted state it is the elevated FFA's that stimulate basal insulin secretion.
(3) Free fatty acids are not dietary fat. I think this is a common misconception and a misguided rationale for advocating a low fat diet. As MM points out, my concerns are that FFA's remain elevated throughout the day on VLC diets. http://carbsanity.blogspot.com/2011/04/adipose-tissue-as-buffer-for-daily.html
We may not want fat to be trapped, but every cell in our body wants it to be, it seems.
(4) Yeah, it is often the case where what we might consider elevated blood glucose is described as "normoglycemic". Still, not horribly elevated.
@MM: At this point I would never consider eating a VLC diet for maintenance. I am open to using it as a weight loss tool, but IMO, yes, my opinion folks for whatever it's worth, it's a trap for the long term. I've compromised my health enough over the years.
It's kinda like how some view religion and going to Heaven. If those of faith are wrong, they lose nothing. If those w/o faith are wrong, they stand to lose a lot. If I'm wrong about NEFA, I don't see how eating a real whole foods normal diet like those consumed by the vast majority of humans for millenia can harm me. If I'm right, I go to an early grave and don't even get the ambulance ride to the hospital.
Do you have a reference for that? I'd prefer it if the study was done in humans.
http://carbsanity.blogspot.com/2010/10/insulin-resistance-taubes-v-frayn.html
Adipose tissue and the insulin resistance syndrome
As adipocytes get larger they become more resistant to insulin which is why the obese have elevated circulating NEFA on average compared to lean. Obviously there's more than just this as a high percentage of obese do not become diabetic.
http://diabetes.diabetesjournals.org/content/47/10/1609.full.pdf
Yes, I agree VLC for maintenance looks like a bad idea. I think I'm definitely better off for giving up on VLC. I've lost 13 pounds (so far) when, if you remember, I plateaued on VLC for a year and a half. I can't thank you enough for helping me realize VLC was not the end-all be-all of human nutrition. I definitely agree with "moderation in all things" even if it sounds corny. :)
Here's an inpatient study where obese type 2 diabetics were placed on a 20 gram/day low-carbohydrate diet for two weeks. After two weeks on low-carb, insulin sensitivity
improved by approximately 75%.
Knowing what I know now, were I diabetic, I would do VLC to lose the weight, and gradually ease into a more moderate carb consumption for maintenance. Where low carbers get in trouble is they seem so intent that dietary fat has no impact on weight they fail to lower it even modestly to accomodate increased carb intake.
I see type 1 diabetes being caused by beta cell dysfunction. Although these people will often develop insulin resistance from exogenous insulin administration, beta cell failure is the first cause.
However, type 2 seems more complex, involving sequential insulin resistance in the skeletal muscles, the liver, the brain, the pancreas and finally in adipose tissue. Once the liver becomes insulin resistant and is unable to shut off gluconeogenesis, there may be a progression into a drug-dependent state if the pancreas is unable to compensate with enough additional insulin secretion. And because insulin also acts as a growth factor, even if the patient does not develop overt diabetes high insulin levels can generate their own problems in the circulatory system.
In case it's of interest to you, I did a blogpost a while ago that surprised me. In people who are not yet insulin resistant, high-carb/low-fat may actually work better for weight loss: Insulin Sensitivity Affects Weight Loss.
http://lowcarb4u.blogspot.com/2010/10/insulin-sensitivity-affects-weight-loss.html
I've read that blog post and the studies. I have some thoughts on why this might be the case, but, as usual for me, I've gotten sidetracked a bit.
High protein diets work pretty darned well ad libitum for weight loss (30% protein/20 fat/50 carb), and high protein relatively low carb (20-30% carb) on weight maintaining diet have been effective in improving glycemic control.
I used VLC to lose weight pretty fast and furious. I eat more carbs these days as a result of everything I've read for maintenance. Were I diabetic, I would probably do VLC to lose the weight ... a diet that would not necessarily have to be high fat by absolute content and not limit protein.
The progression of pathological IR described in the literature (that Frayn paper and http://carbsanity.blogspot.com/2010/07/progression-of-insulin-resistance.html for example) seems opposite of what you describe.
My personal bias is that insulin levels should be lowered by improving sensitivity to its action. Over the long term at maintenance levels, VLC must be VHF and as far as I'm concerned, someone who wasn't diabetic to begin with who can't handle eating a potato after a decade or more eating this way is "diabetic" whether or not they ever exhibit hyperglycemia. It's only half of the picture, if that! This is, of course my opinion, but there is a TON of peer review literature consistent with this.
As far as adipose insulin resistance being first, the knock-out studies by C. Ron Kahn et. al, would demonstrate that when insulin resistance develops in adipose, it becomes difficult to gain weight.
This is an interview with Dr. Kahn, but he mentions knocking out the insulin receptor in fat tissues in one of the questions.
http://archive.sciencewatch.com/may-june2005/sw_may-june2005_page3.htm
covered calls is the technical name for this strategy) & combine this with a Real Estate portfolio designed to give
you capital growth (he focuses on how to get ‘no money down’ deals).
With the "information age" well under way, this is not surprising.
You also need to know how you are going to publish your book and you have 3 choices:
.
my blog free Pdf ebook download
Post a Comment
Comment Moderation is ON ... I will NOT be routinely reviewing or publishing comments at this time..