When Insulin Goes Away ...
The very core of LC theory on weight loss is that insulin *causes* fat accumulation through it's action, and basically if we can lower insulin, weight loss occurs. Sounds simple and straight forward.
From Guyton & Hall's Textbook of Medical Physiology, 11th Edition, p. 966
Insulin Deficiency Increases Use of Fat for Energy
All aspects of fat breakdown and use for providing energy are greatly enhanced in the absence of insulin. This occurs even normally between meals when secretion of insulin is minimal, but it becomes extreme in diabetes mellitus when secretion of insulin is almost zero. The resulting effects are as follows.
Insulin Deficiency Causes Lipolysis of Storage Fat and Release of Free Fatty Acids.
In the absence of insulin, all the effects of insulin noted earlier that cause storage of fat are reversed. The most important effect is that the enzyme hormone-sensitive lipase in the fat cells becomes strongly activated. This causes hydrolysis of the stored triglycerides, releasing large quantities of fatty acids and glycerol into the circulating blood. Consequently, the plasma concentration of free fatty acids begins to rise within minutes. This free fatty acid then becomes the main energy substrate used by essentially all tissues of the body besides the brain.
Wow! This is what Gary Taubes and all the LC gurus have been telling us for years now! All along our fat cells have been hoarding these fatty acids and starving our cells of their energy so we go around hungry all the time. Of course most of my readers know this to be the stuff of pure fantasy. In a human with a functioning pancreas, baseline insulin becomes elevated in direct response to increased adiposity to keep from circulating free fatty acid levels in check.
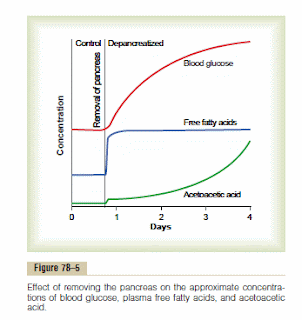 On that same page we find the graphic to the right. (I was thumbing through my 6th edition hard copy and came across this just this morning). Unfortunately, I cannot find the direct reference from which to get actual concentrations/levels, but that isn't really the point. This was what happened when some animal was depancreatized (species not specified either) thereby causing insulin to drop to zero. The first thing that goes awry? The fatty acids. Within minutes. The fatty acids jump right up there and remain elevated. Hyperglycemia develops but levels off. Ketone production is more delayed, slow at first but then accelerates. Note that ketones are produced from the excess fatty acids. From the text:
On that same page we find the graphic to the right. (I was thumbing through my 6th edition hard copy and came across this just this morning). Unfortunately, I cannot find the direct reference from which to get actual concentrations/levels, but that isn't really the point. This was what happened when some animal was depancreatized (species not specified either) thereby causing insulin to drop to zero. The first thing that goes awry? The fatty acids. Within minutes. The fatty acids jump right up there and remain elevated. Hyperglycemia develops but levels off. Ketone production is more delayed, slow at first but then accelerates. Note that ketones are produced from the excess fatty acids. From the text:Excess Usage of Fats During Insulin Lack Causes Ketosis and Acidosis.
Insulin lack also causes excessive amounts of acetoacetic acid to be formed in the liver cells. This results from the following effect: In the absence of insulin but in the presence of excess fatty acids in the liver cells, the carnitine transport mechanism for transporting fatty acids into the mitochondria becomes increasingly activated. In the mitochondria, beta oxidation of the fatty acids then proceeds very rapidly, releasing extreme amounts of acetyl-CoA.A large part of this excess acetyl-CoA is then condensed to form acetoacetic acid, which in turn is released into the circulating blood. Most of this passes to the peripheral cells, where it is again converted into acetyl-CoA and used for energy in the usual manner.
At the same time, the absence of insulin also depresses the utilization of acetoacetic acid in the peripheral tissues. Thus, so much acetoacetic acid is released from the liver that it cannot all be metabolized by the tissues.Therefore, as shown in Figure 78–5, its concentration rises during the days after cessation of insulin secretion, sometimes reaching concentrations of 10 mEq/L or more, which is a severe state of body fluid acidosis.
As explained in Chapter 68, some of the acetoacetic acid is also converted into b-hydroxybutyric acid and acetone. These two substances, along with the acetoacetic acid, are called ketone bodies, and their presence in large quantities in the body fluids is called ketosis. We see later that in severe diabetes the acetoacetic acid and the b-hydroxybutyric acid can cause severe acidosis and coma, which often leads to death.
No ... I'm not going to equate benign ketosis with severe ketoacidosis, but I am going to point out a few things that these short excerpts from a (renowned) physiology text illustrate.
Anything taken out of context can be used to support
a flawed theory on the basis of scientific *facts*
This is what Gary Taubes is so clever at doing. Nobody denies the basic physiological facts that insulin's actions on the adipocyte are to promote fat storage not mobilization. Nobody. But you would have to ask yourself is how it was that the 98-99% of the population with normally functioning pancreata during most of their lives used to manage to remain normal weight, in large proportions, for centuries on end, despite all that Neolithic carbohydrate consumption and corresponding insulin spiking?
I imagine many low carbers gleefully read "this free fatty acid then becomes the main energy substrate used by essentially all tissues of the body" and started breaking out into "I'm a fat burner" chants and cheers. But you will notice that NEFA/FFA become the main energy substrate -- that means burning less glucose and more fat as a proportion. There is NO indication that the body burns more energy in total. And herein lies the problem. Because what you see with these depancreatized animals is that as fatty acid's remain elevated and displace glucose as substrates glucose levels build but so do ketones. Ketones are not being used as energy in this scenario because the animal is awash in its free fatty acids.
Now, thankfully LC diets do not achieve the serious depression in insulin levels that are claimed so as to actually cause this deadly scenario. However, the graphic above shows that the NEFA/FFA levels are the first to go awry and these essentially cause the other two.
But insulin's action to sequester fatty acids in the adipose tissue is not only "healthy" but ultimately required to sustain life. We don't want it going away any time soon. Rather than obsessing over the bulging thighs of an unfortunate Type 1 diabetic who injected herself too frequently in the same locations, one should take note of what would happen to the poor woman were she not to have injected at all. Really folks, otherwise, why not just partially pancreatize the obese and be done with it?
Comments
I'm not impressed with (a) the basis of her hypothesis nor (b) her insulin stimulation data. That's as far as I've gotten so far.
(a) She cites insulin infusions -> IR which is not analogous to any physiological phenomenon except something like an insulinoma. The other study is dubious at best, that lowering insulin secretion on a hypocaloric diet seemingly resulted in greater fat loss (but not weight loss) in a small human study. Still, if one accepts that her observations support a hyperinsulinemia -> IR/beta cell dysfunction model ...
(b) Monoglycerides: She acknowledges that 25 micromolar elicited an insulin response at basal glucose levels. Well, free fatty acids contribute to an insulin response. Also she says she can't find where that concentration compares to normal circulating levels. Fair enough, but if they are present in (her words) small quantities in foods, one could find out the content in the food and do a rough calculation of concentration were all to be absorbed intact and dispersed in our roughly 5L of blood.
She continues on to the artificial sweeteners and acknowledges that "interestingly" only saccharin elicited the response at physiologically *possible* levels -- e.g. with high consumption levels. Ummmm.... This lady looks old enough to realize that except for the occasional pink packets this AS practically disappeared from the market by the late 80's ... right? I mean, several years back you could still get the original Tab cola with it but now even that has aspartame (and the original was different ... I miss the occasional Tab!).
...
More when I can devote "screen time" to trying to watch the slides available with the lecture. I'm left frustrated at this at the moment.
No, my core reason for avoiding carbs is by eating lowfat proteins to satiation, I have less calories AVAILABLE FOR STORAGE because so many of the protein calories get burned up in converting most of the rest of the protein into glucose, in my liver.
Healthy populations have (very) low basal insulin and excellent glucose tolerance. They have good insulin signaling. These folks are almost never on a low carb diet, but they mostly have no access to certain neolithic foods. Very low carbers might have low basal insulin (by the way, I have not seen this established), but they are glucose intolerant. This is explained away by down regulation of enzymes, but I suspect it is a result of physiologic insulin resistance. I still don't know if this is 'less than optimal'.
plasma; opalescent,it stayed that way when left for 24 hours. Trigs, LDL both sky high, TC was above 8mmol/l.
I assume it was something like this
http://www.duke.edu/~ema5/Golian/Slides/1.1/CardioImages1.html
Two months later plasma,clear straw coloured and TC was 3.2 mmol.
The difference was of course insulin.
And I couple that with observation that, near as I can tell, when LC diets are successful for glycemic control it is for a period when the subjects are following the diet. But many long term low carbers do NOT have good glucose tolerance. Since this was a weight loss study it's hard to say what the NEFA profile would look like or if the elevated levels are a problem (they don't seem to be in caloric deficit), but at some point ...
A study I would like to see is what happens to a group of low carbers "cured" by Westman's diet transitioned to a "normal" diet for 3 months and how many remain non-diabetic. There are studies showing that post GBP surgery, that "crash diet" and aggressive insulin treatment when returning to normal diet, glycemic control is retained. I think the fatty acids are the likely culprit for why low carbers don't seem to enjoy similar glucose tolerance.
In my view, LC doesn't achieve the T1 state. Thankfully the "all or nothing" extreme of the dogma is neither true nor achievable. But, in the long term the insulin resistance remains or gets worse.
Lately I'm seeing another "make shit up" trend to describe physiological IR as "the good kind". Have you seen any evidence of that? I've not. Transient IR is glucose sparing in times of scarcity. But humans have adapted to carbohydrate abundance for thousands of years and I still see little evidence for the extreme interpretation that our paleo ancestors were VLC/HF near-carnivores.
A high fat VLC'er consuming <20g/day can become a moderate fat mod-carber with only a few changes. Using a NAD, simply substituting around 1c. rice for 2T commercial soybean-oil based creamy salad dressing is enough to keep one out of ketosis (ketones seem to impact the beta cell as well) and perhaps eating a little more salmon or even white fish instead of 70% ground beef to get a bit more energy from protein may be all it takes.
So I guess my point is that the lowish carb diet can even seem pretty "extreme" vs. the SAD (I'd say the SAD is the absurd extreme!) even though it seemingly resembles the VLC diet, it doesn't take much for it to be a wholly different metabolic animal.
BTW: Other than the Inuit, what are the very high fat cultures I'm not aware of?
Thanks to Beth's link, I was reminded about the whole obesity and BCAA thing too ... and Guyton provides a theory there ;)
I thought I would mention that the fountain versions of Diet Pepsi and Diet Coke contain a sweetener mix of AceK, aspartame and saccharin. I don't know if it makes a difference or not but saccharin is still out there lurking about.
Is this question directed to me? I didn't mention such a culture. I'm not aware of an extremely healthy, long living lchf population. The traditional Inuit had their problems.
On Inuit and CHD.
@Frosty: Welcome! I was not aware that fountain sodas may contain some saccharin. It wasn't all that long ago that they were allowed to remove the warning label for this AS so I find it surprising that this would not be common knowledge. Still, it's not prevalent in a lot of products and Corker used wording indicating levels possible with high consumption.
Related note: She highlights an experiment of the effects of 48 hour chronic exposure to saccharin. I'm baffled by even conducting this experiment as it would not have the most remote physiological applicability. Even in my Tab-addict days I wouldn't drink it constantly for 24 hours!
I'm really perplexed by this researcher. Her name is on various papers on beta cell function from the 90's discussing FFA's and the long chain CoA's, etc. How she went from there to looking at a laundry list of possible environmental chemical agents -- many of which would would likely be higher in reducing diets that have been known to reverse diabetes in some -- that cause basal hyperinsulinemia. It's hard to tell, but it seems like they're incubating cells with fasting glucose levels and adding these various agents to see if there's an insulin response. It's portrayed as a "basal insulin response" even though if it were a dietary agent, any insulin response would be a -- likely very minor -- postprandial spike. The whole hypothesis and model seems rather bizarre to me. Now FFA? These ARE elevated normally in the fasted state and have been shown to contribute to fasting insulin levels.
http://diabetes-editor.blogspot.com/2011/06/banting-memorial-lecture-2011.html
http://www.solvingdiabetes.org/2011/07/20/highlights-of-ada-scientific-sessions-ii-2011-banting-lecture/
Hormone-Sensitive Lipase Has a Role in Lipid Signaling for Insulin Secretion but Is Nonessential for the Incretin Action of Glucagon-Like Peptide 1
"The free fatty acid (FFA) supply to the pancreatic β-cell is of importance to both its normal function and its failure in type 2 diabetes (1,2). Fatty acid deprivation causes a loss of glucose-stimulated insulin secretion (GSIS), a process rapidly reversed by replacement with exogenous FFAs (3). In contrast, elevated levels of FFAs supply augment GSIS (4); however, if the excess is chronic, particularly in association with elevated glucose (5), it can induce β-cell apoptosis (1,2,5,6). Although the mechanisms involved in FFA modulation of insulin secretion and β-cell toxicity are not well understood, it is increasingly apparent that the intracellular metabolism of FFAs, resulting in the synthesis of lipid-signaling molecules such as diacylglycerols (DAGs) (1,7) and the accumulation of toxic lipid species such as ceramide (8,9), is involved.
The fatty acid supply to the β-cell can be from exogenous sources such as plasma FFAs"
Yesterday, after reading Ned Kock's Aug. 1 post asserting that “abnormally elevated insulin is associated with body fat accumulation,” I first thought of GT, of course, then I thought to myself (as I always do nowadays whenever I read other bloggers' statements regarding insulin), “I wonder what Carbsane would have to say about this?” and then I drifted off into my own, highly amateurish, speculations regarding association and causation. Last night, the instant I saw “When Insulin Goes Away,” I thought, “Hmmm. Coincidence?” all of which was mostly clarified upon seeing your comments at Ned's site this morning. While you were very diplomatic and Socratic in your final question to Ned, I came away wondering what you *really* might have been inclined to say. I have a couple of specific questions, however, that I hope you'll be willing to respond to directly. These are germane to both the insulin-response considerations and your discussions of substrate balance/fate of nutrients, etc.
Ned says that he lowers calories and carbs on days that he is not working out. The lowering of calories already adds a confounder, but I'm always running into "gurus" and bloggers that recommend adjusting carbs to level of activity, either on a daily basis as Ned specifies, or in general as someone like A. Colpo recommends. What is your take on this? Isn't the metabolism perfectly capable of averaging out variations in ingested nutrient composition? Further, for example, if an inactive or less active person desires to consume a high-CHO diet, what difference does it make as long as appropriate adjustments are made to quantity of fat, and possibly protein, consumed?
I, myself, adjust my consumption of carbs but based on an entirely different consideration from "fat-trapping" or insulin-spiking and entirely unrelated to my activity level. Because CHO+fat tends to be a huge cue for me to overeat, on days when I've planned higher fat consumption (for example, when I'm going to have my beloved chuck roast -- even though I de-fat it to a fare-thee-well!) I drastically reduce or eliminate the carbs (for example my almost equally beloved baked potatoes). On days when I've planned for minimal fat consumption, I go crazy with those aforementioned potatoes (love 'em hot or cold; eat 'em like a candy bar!). In other words, it's strictly a behavior-modification technique, and I assume that everything averages out and that I'll still have plenty of glycogen (and fat!) for my insanely long bicycle rides even though they may occur on low-carb days. So, what am I missing or doing wrong?
Thanks CarbSane. Your blog is essential reading!
Regards,
Archie
I think when you outline your approach vs. Ned's it highlights an individuality. My own approach is similar to yours but I even do it mealwise -- if I'm eating carbs I go easy on the fat and vice versa, and if I'm going to eat a high carb/fat meal, I am much more mindful of portioning out. I do this because I don't believe we're well adapted to mixed meals to sense the calorie dense C&F foods.
Humans seem perfectly capable of averaging things out. Many will be in slight surplus one day, slight deficit the next. Ned's approach seems to me to be an attempt to be closer to in balance at all times. But I delved into meal timing and size a while ago and the bottom line seemed to be that for most of us it makes not a darned bit of difference whether we eat a 2000 cal in the morning, or as 2 1000 cal meals or 3 600 cal meals + 200 cal snack or even 10 mini-200 cal meals. It matters not if we eat 2000 cal at 11am or 11pm, just upon rising or just before going to sleep. So all of the meal timing/sizing strategies that work do so b/c they prevent overeating.
Seems Ned's approach may be that on days he burns more he eats more and that more is in the form of carbs (and their coincident cals), on days he expends less he eats less (and thus doesn't add the extra carbs/cals). Sounds like a well reasoned approach to me.
In the end, it's long range energy balance that matters. However I would say that if one is averaging out longer term swings a problem can be created. The body doesn't really kick into adapto-mode until 48-72 hrs of major calorie restriction so if it's reasonable fluctuations on a day to day or couple of day basis, this should be the same as a constant intake. But if someone is crash dieting (1500 cal deficit) for a month then binging for a month (1500 cal surplus), they will probably end up fatter because the body will dial back BMR in response to the longer term/severe CR and not recoup so that 1500 cal surplus from baseline may now be 1800 cal. Hope that makes sense.
Thanks for reading and your kind comments Archie!
___________________
There's more to this issue than you're discussing here. Some things to consider
** muscle anabolism surrounding workouts
** muscle glycogen replenishment
Both of these effects suggest one should raise calories, and research shows carbohydrates eaten here are very beneficial.
But if all one did was raise calories (fat and carbohydrate) and just ate more the possibility of over-consuming becomes greater. It's much easier to reach 6,000 calories with baklava and ice cream than with boiled potato or rice.
So as a matter of compliance it makes a lot of sense to restrict fat on high calorie days and let fat content rise a bit on low calorie days.
So you can see there's a confluence of factors that combine to form the rules - there are "rules" suggested by sports performance and biochemistry research (raise carbohydrates and calories around workouts) and rules suggested by psychological research (you're more likely to overconsume calories on high fat when you're not paying attention).
and if you find carbohydrate rewarding, eating a lot of carbohydrate around workouts reinforces working out.
That's off the top of my head ... (haven't even written about insulin sensitivity issues) there's lots of research pushing this strategy (high carb, high calorie, low fat on workout days, raise fat in a controlled manner on non-workout days.)
"count calories or use rules, tricks and strategies that count calories for you (realizing that human nature cuts corners around restrictions, so keep the calorie counting available as a back up)
If your LDL is low, and HDL high, does that mean NEFA is in check?
I ask, because I have mild diabetes, but low trigs. Had low trigs and low LDL on HF/HC (Weston A. Price) type diet. Had low trigs and low LDL on semi-vegetarian normal-person diet. Had low trigs and low LDL on low-carb diet. Haven't had these tested since going on moderate-carb, lowish-fat diet.
Since I have elevated glucose and low trigs, should I worry about NEFA? This NEFA thing is new to me. I have, throughout this time, ranged from very slightly chub to pretty thin.
"count calories or use rules, tricks and strategies that count calories for you (realizing that human nature cuts corners around restrictions, so keep the calorie counting available as a back up)
Sounds right! I would add that for the obese or highly IR individual, consuming large mixed meals is probably not as good for one's body compared to spacing and separating. I say this because both of these types have "plasma clearance issues". Calories will balance, but elevated circulating glucose and fatty acids are another issue.
I'm just regarded as a mildly confusing, mildly diabetic Type II. I think my BG is too "good" (6.0-6.4 A1C) for any medical person to get concerned. (My doctor is not Dr. Davis!) I requested, and got (somewhat miraculously) genetic testing for MODY 2, which fits this profile (low trigs, mild diabetes, not associated w/ overweight, though MODY 2's can be overweight), but was negative. There's at least one even rarer MODY that's similar, but until I have a few thousand $ to spare for another genetic test (or until you can have them done at the mall), I probably won't know. It also could be something idiosyncratic that behaves the same way. Or... something else.
Post a Comment
Comment Moderation is ON ... I will NOT be routinely reviewing or publishing comments at this time..