You're as Hyperinsulinemic as You Need to Be †
Recent comments by Todd Becker (Getting Stronger blog) have prompted me to write yet another post on the (infamous on this blog) Grey & Kipnis study. Perhaps, part of the problem in discussing these issues is a failure to define what it is we're talking about. For instance with IR, we have chronic/pathologic IR, glucose-sparing IR (physiologic, fasting/carb restriction), and postprandial IR (usually impaired glucose clearance following a high fat meal or large fructose load). With hyperinsulinemia we can talk about basal insulin levels vs. postprandial insulin levels. It appears to me, that if we combine the observations in G&K with those of the long term fasting study, with the hypothesis of G&K -- that diet can play a role in basal hyperinsulinemia and therefore contribute to obesity -- perhaps basal insulin levels are comprised of both a chronic component (I would suggest related to NEFA) and a more transient component due to the diet of the previous day(s).
So, Todd wrote: Forgive me if I oversimplify the argument in your above post:
1. Obesity leads to spilling of excess fat as NEFA.
2. Excess NEFA leads to insulin resistance in the tissues, including the adipocytes
This mis-states the critical point of the insulin resistance in the adipocytes occurring first. When we get obese we overfill cells, and larger more mature adipocytes have been shown to be less sensitive to insulin (while retaining or perhaps getting even more sensitive to ASP - Allan Sniderman et.al., blogged on here)
Of particular importance, as adipocytes differentiate and become larger with greater triacylglycerol synthetic capacity and triacylglycerol mass, they become more responsive to ASP ... in contrast with the response to insulin, larger adipocytes display no evidence of resistance to the stimulatory effects of ASP on triacylglycerol. (Cites Walsh et.al. - Sniderman amongst -- abstract only, if anyone has full text I'd appreciate a copy!)
Since ASP is a more potent stimulator of triglyceride formation in adipose tissue while insulin is almost certainly the dominant factor in lipolysis/mobilization by HSL (there is some evidence for a suppressive role for ASP too, but minor compared to insulin) this presents a vicious cycle. It also explains how an IR individual can continue to get fatter and fatter in the face of dwindling sensitivity to insulin.
3. Elevated NEFA stimulates the pancreas to produce elevate insulin as a feedback response to minimize lipotoxicity, one manifestation of which is insulin resistance.
In short, obesity causes hyperlipemia and compensatory hyperinsulinemia.
But none of this is incompatible with the additional observation that consumption of a high carbohydrate diet tends to raise insulin levels more readily than low carbohydrate diets of equal calories, and is itself a path to hyperinsulinemia and obesity. One of the key findings of Grey & Kipnis ... was precisely to establish this point.
I agree with point 3 (with the clarification that the resultant IR we're talking about is peripheral/skeletal muscle IR but the primary goal is to suppress lipolysis to lower NEFA), and with the compensatory clause. I would go so far as to agree that this etiology of chronic hyperinsulinemia is not incompatible with the notion that postprandial effects could also be true and/or compound matters. Where I disagree is that the results of G&K did not establish this as a pathway to obesity.
G&K ultimately fails to support TWICHOO because:
G&K ultimately fails to support TWICHOO because:
- Evidence must be produced to support that carbs → hyperinsulinemia in and of themselves. This would require repeating this study with subjects with normal insulin levels to see if changes in diet composition (at weight stable calories) would produce sustained hyperinsulinemia.
- However, at isocaloric levels, even if hyperinsulinemia resulted, weight would have to be gained to support the hypothesis ...
CATCH 22
- The LFHC diet used was not exemplary of even the fattening SAD. This is one thing TWICHOO proponents are unfortunately hypocritical about. When an LC diet is not sufficiently low in carbs, studies are routinely dismissed or criticized (rightly so in most cases) for that fact. This study used an extreme diet that does not remotely resemble the macronutrient content of the typical obese person. Therefore even had the subjects gained weight, it would be a stretch to conclude that carbs were the reason they became obese.
- To support the causal direction of carbs → insulin → obesity, the study would have had to start with lean subjects with normal insulin levels. Then demonstrate that hyperinsulinemia could (a) be induced by change in diet in these folks, and (b) result in weight gain at isocaloric levels.
Still, that the already obese subjects did not become more obese in the face of skyrocketing insulin levels, or less obese in the face of plummeting ones, on isocaloric diets is rather damning of TWICHOO. As is the significant weight loss achieved with caloric restriction despite extreme manipulations of dietary composition producing wild fluctuations in insulin levels.
G&K stated:
"The hyperinsulinemia characteristic of obesity has generally been regarded as a compensatory adaptation to the peripheral insulin antagonism that has been demonstrated in obesity...The results of our studies suggest an additional explanation for the hyperinsulinemia of obesity...In the markedly obese person, not only is the total caloric intake typically increased, but the absolute amount of CHO ingested is also appreciably greater than normal...In view of these considerations, it seems reasonable to suggest that the hyperinsulinemia of obesity is also a result of dietary factors rather than exclusively a secondary adaptation to insulin resistance. In this context, the insulin antagonism that develops with obesity might actually represent an adaptive mechanism to protect the obese person from hypoglycemic episodes resulting from the excessive intake of carbohydrate.”
I am very confused by that last statement. Are they suggesting that peripheral IR is what is induced by excessive carb intake to protect the individual from having a hypoglycemic attack thus leading to hyperinsulinemia? That might seem plausible, but it's not what they said, which was that hyperinsulinemia was a protective adaptation against hypoglycemia. Perhaps transient IR induced by fructose is somewhat protective? Makes little sense to me, however. We have a metabolic protection against hypoglycemia for that other nutrient that elicits an insulin response, protein, which stimulates glucagon which stimulates glucose production by the liver to protect against hypoglycemia. Protein ingestion promotes insulin sensitivity (see here)
Once established, chronic hyperinsulinemia does compound insulin resistance by keeping glycogen stores topped off, thus reducing non-oxidative glucose disposal (NOGD). A defect (reduction in) NOGD is the most common factor to which peripheral skeletal muscle IR is attributed.
The sentence I bolded in the above excerpt is routinely criticized. Of course absolute amount of CHO ingested is greater than normal in a hypercaloric SAD-style diet. What is this based on? How about let's take a look at the data from the study itself!
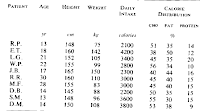
At right is the baseline data from G&K {click to enlarge}. I have put this into a spreadsheet and also calculated the macronutrient intake in grams. Below left I've plotted carb intake as % total intake (vertical) vs. starting weight (horizontal). There is a statistically significant (r = 0.673, P<0.05) negative correlation between weight and carbohydrate as percent of intake. Below right, although a slightly negatively sloping trendline can be generated by the computer, we see essentially no** correlation (r = 0.04) between absolute carbohydrate intake and weight.
** r values are correlation coefficients that vary from 0 to 1. For r=0, there is no correlation, for r=1 there is a perfect correlation (every data point falls on the line).
And there you have it folks. The more obese the subject, the lesser the carb consumption as a percentage of intake, but it's a wash in terms of total grams. But I suppose this does lend credence to Sisson's 150g rule for insidious weight gain.
So I'll conclude here by addressing the rest of Todd's comment:
G&K themselves note that, while the insulin levels of their obese individuals remained elevated even after dietary intervention, three to four weeks is simply insufficient to effect a significant reduction in insulin levels. However, they state:
“When insulin secretion has been studied in obese subjects before and after more prolonged periods of caloric restriction (two to three months) or after normal weight has been attained, normal basal plasma insulin levels and insulin secretory responses to glucose have been observed…Consequently, it cannot be ascertained whether restoration of normal insulin secretion was a consequence of CHO restriction or the disappearance of insulin resistance.”No, it can't be ascertained by this study. However weight loss on any manner of diets restoring normal insulin signaling seems to have been demonstrated many times in the intervening four decades since G&K's study was performed. In the end, G&K is a quintessential masterpiece for obliterating Taubes' latest version of TWICHOO -- that being that carb restriction and not calorie restriction is what produces weight loss. But it is severely limited in telling us anything about insulin and insulin resistance. It's a good thing scientists have been toiling away ever since to answer those questions and seem to have made significant progress in solving this puzzle.
Exactly my point. It doesn’t have to be “either/or”, it can be “both/and”. The debate about whether
I. A high carbohydrate diet drives hyperinsulinemia drives insulin resistance drives obesity, or rather
II. Positive caloric balance drive obesity drives insulin resistance drives hyperinsulinemia drives an appetite for carbohydrates
seems so much like the debate about whether the chicken came before the egg. As with much in biology, causation occurs in circles. Why can't both I and II be true?
They both can be true, but the evidence is not there to support I Todd. (I also take exception with the last part about driving appetite for carbs in II).
Other than the pleasure of slaying dragons, why is it so important to “defeat” the carbohydrate/insulin hypothesis, rather than to fill it out by highlighting the complementary half of the circle of obesity?
Because I don't see any evidence that it is correct, and I think propagating false information is damaging to progress. There is merit to carbohydrate restriction in the face of rampant hyperinsulinemia as well as evidence for LC diets being more effective in practice for those with IR (including T2's). I hope to put some of my thoughts on why this is to "paper" in the near future. There is merit to the notion that once someone IS in such as state, carbohydrates exacerbate the situation (the other half of that circle that only comes at the top of a lollipop perhaps).
I think what you are doing on your blog is better seen as a valuable and insightful corrective to an otherwise incomplete theory, rather than as a revolution that topples it.
You're certainly entitled to your opinion and I'm always welcome to you sharing your thougts even if we disagree on most things it seems. However I believe TWICHOO is *wrong* and needs toppling. Thus I'm hopeful that at some point we can indeed topple Taubes' horrific cherry picked version of the science of obesity. Carbohydrates do not cause obesity. That is his dug-in-heels message in WWGF. Billions of humans demonstrate otherwise.
† The title of this post is a take on the pseudoscience of Tom "Fat Head" Naughton who created a whole new theory on how we are as fat as we need to be to maintain normal blood sugar. This is based on the flawed notion that fat cells remain insulin sensitive to the bitter end. The reverse is overwhelmingly demonstrated to be true by the research of actual scientists. Basal insulin levels increase in response to increased fat stores as the body's protective mechanism to ensure fat is stored in its proper place. Therefore you are as hyperinsulinemic as you need to be to maintain normal circulating NEFA levels and protect against lipotoxicity (and downstream hyperglycemia, glucotoxicity and glucolipotoxicity).


Comments
CS: Since ASP is a more potent stimulator of triglyceride formation in adipose tissue while insulin is almost certainly the dominant factor in lipolysis/mobilization by HSL (there is some evidence for a suppressive role for ASP too, but minor compared to insulin) this presents a vicious cycle. It also explains how an IR individual can continue to get fatter and fatter in the face of dwindling sensitivity to insulin.
TB: I agree with you that “insulin is almost certainly the dominant factor in lipolysis/mobilization by HSL”. But what does this say about the obese G&K subjects who lost weight on hypocaloric diets, given their elevated insulin levels? That would tend to suggest their apparent weight loss may not have been mainly fat loss, wouldn’t you say? Or if there was fat loss, it was only because their calorie was so low that even the trickle of meager fat loss was sufficient to make a difference when they stood on the scale.
CS: G&K ultimately fails to support TWICHOO because:
• Evidence must be produced to support that carbs → hyperinsulinemia in and of themselves. This would require repeating this study with subjects with normal insulin levels to see if changes in diet composition (at weight stable calories) would produce sustained hyperinsulinemia.
• However, at isocaloric levels, even if hyperinsulinemia resulted, weight would have to be gained to support the hypothesis ...
• To support the causal direction of carbs → insulin → obesity, the study would have had to start with lean subjects with normal insulin levels. Then demonstrate that hyperinsulinemia could (a) be induced by change in diet in these folks, and (b) result in weight gain at isocaloric levels.
TB: I don’t accept your postulate that the carbohydrate/insulin hypothesis must show carbs by themselves initiating hyperinsulinemia and obesity! The C/I hypothesis states only that carbohydrates are a necessary or likely precondition – not that they are a sufficient condition. I don’t believe I’ve seen that Taubes or anyone else ever claims carbs are a sufficient condition for obesity . They are a potent fuel that accelerates the fire, not the tinder and oxygen or the whole fire itself.
We need only demonstrate something much weaker, namely that high carbohydrate consumption sustains hyperinsulinemia and obesity and tends to prevent their reversal. That would show that carbs are a necessary (or at least highly contributory) precondition. (I make this caveat to allow for other routes such as high fat + ASP). Thus, studies in insulin resistant, overweight or obese individuals are actually more relevant than the studies in lean, insulin sensitive individuals that you are asking for.
The fact that lean, fit, insulin sensitive individuals don’t have a problem with carbohydrate does not contradict the C/I hypothesis! The C/I hypothesis does not claim that high carbohydrate are a sufficient condition for hyperinsulinemia or obesity. So the high-carb Kitavans, Japanese, etc. are not counterexamples, probably because they are insulin sensitive. It is likely that the C/I hypothesis only applies once you have some degree of insulin resistance. Obesity may have multiple necessary (or contributory) conditions, only one of which is carbohydrates. But, in an environment where many of those other conditions (inflammation, sedentariness, stress) etc. are present, carbohydrates may be particularly important.
It may be that carbs don’t initiate IR, but perhaps they make it worse, in the following way: Typical slightly overweight Americans fluctuate in weight and degree of insulin resistance. If high carbohydrate consumption only act to inhibit the “downcycle”, favoring the “upcycle” of adiposity, that is all that’s needed to change the normal oscillatory trajectory, favoring weight gain and inhibiting weight loss. If that is true, then by decreasing the inhibition of lipolysis, for more hours in the day, low carb diet can help such folks lose weight, even if a high carb diet by itself (with no IR) does not turn lean people into fat people.
The key question is: If you are already moderately insulin resistant and hyperinsulinemic, will a high carb diet tend to keep you that way or worsen the trajectory? I think the answer is yes, for several reasons:
A. Insulin resistance tends to increase the insulin response to carbs (up until one becomes Type II diabetic). This reinforces hyperinsulinemia, by never letting insulin levels drop down long enough for sustained lipolysis to occur.
B. Whether or not the insulin is efficient at storing new fat is not so important, as long as elevated insulin levels inhibit significant net lipolysis. While fat loss is not prevented in this condition, it is inhibited – which maintains obesity and allows it to progress.
C. If you are insulin resistant, carbohydrates tend to promote cravings. While insulin suppresses appetite in the CNS, insulin resistance tends to blunt that response and the peripheral hypoglycemic, hunger inducing effects of insulin can overwhelm the normal CNS satiety response.
According to Velloso and Schwartz (http://www.ncbi.nlm.nih.gov/pubmed/21386802):
“A major and persisting source of confusion surrounding the hypothesis that insulin action in the brain reduces food intake and body weight while also lowering hepatic glucose production and increasing thermogenesis stems from evidence that following systemic insulin administration, the subsequent fall in glucose levels potently increases food intake while also increasing liver glucose production and reducing sympathetically driven thermogenesis. Thus, insulin-induced hypoglycemia potently overrides virtually all of insulin’s central effects, an observation that for many years has confounded research in this field."
There is plenty of evidence that reducing insulin levels can result in fat loss. For example, Woodhouse et al. showed that a reduction in insulin levels is correlated with (and temporally preceeds) weight loss in exercising individuals:
http://www.ncbi.nlm.nih.gov/pubmed/6394195
Now it may be that insulin reduction does not immediately help in all cases. For example, in the obese, presumably IR subjects of G&K, it may be that the insulin levels, though reduced, were still way too high to allow for substantial short term fat loss. As G&K suggest, it may take months for insulin levels to drop sufficiently for both weight loss and restoration of normal insulin sensitivity.
So nothing in G&K contradicts the C/I hypothesis, properly understood as a hypothesis about one important necessary (or highly contributor) condition, not as a hypothesis about the entire etiology of obesity. In particular, it is consistent with many other routes to insulin resistance and hyperinsulinemia. And it may in fact quite unhelpful in explaining the genesis and early phases of insulin resistance. In that sense, carbohydrates are like a fuel added to a fire that gets started for other reasons. But removing the fuel still lets the fire die down. And there may be other fuels (like excessive fat), but I would argue that for many people, carbohydrates are particularly problematic.
Todd
Post a Comment
Comment Moderation is ON ... I will NOT be routinely reviewing or publishing comments at this time..