Mitochondrial Function and Dysfunction
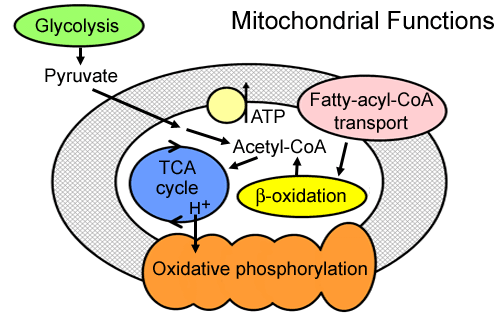
Below is a wonderfully simple depiction of the mitochondria that depicts one of the points I've been trying to raise above the current internet noise about mitochondrial dysfunction. That being that when it comes to carb burning (glycolysis) the initial steps occur outside the mitos while fat burning (ß-oxidation) occurs within the mitos. However both create Acetyl-CoA, and from that point on, metabolism and energy production is the same.
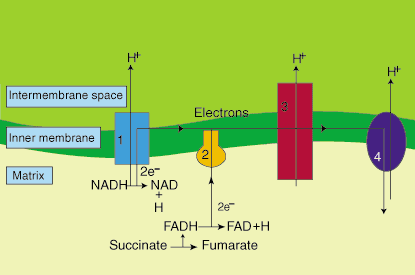
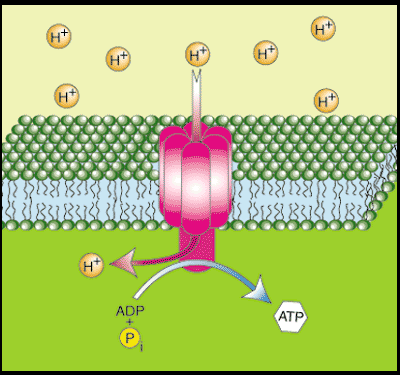
Acetyl-CoA feeds into the TCA cycle, aka the Krebs cycle, producing "reducing equivalents" (NADH and FADH2 , some is formed directly in the ß-ox). These are the H+ shown feeding into that orange "oxidative phosphorylation" worm looking thing. The orange OP is also commonly called the electron transport chain, ETC, and harnesses electrochemical energy to drive the phosphorylation of ADP to ATP.
(For a more detailed but fairly lay-friendly description, the above diagrams come from SparkNotes on cellular respiration)
What stimulates oxidative phosphorylation? INSULIN. Some cites I'll address in the future when I get my grubby cyberhands on the full texts:
Some of the mitochondrial dysfunction is genetic, some acquired. All linked to insulin sensitivity. It's not the action of insulin that is problematic, it is the inability of insulin to act. This post is mostly a precursor to another I hope to post up today on respiratory quotient and longevity. The intent is mostly to drive the point home that all roads (even ketones, which are broken down/converted into Acetyl-CoA) converge to the same path in the mitochondria. So when we're talking proper function or dysfunction, it relates to far more than "fat burning". There appears to be yet another "vicious cycle" emerging here with that chicken-egg debate not far behind. Keeping in mind that the human genome has not undergone en masse mutation since the 1970's to produce the obesity epidemic, however, the notion that mitochondrial dysfunction → obesity is far less likely than obesity → mitochondrial dysfunction. And the dysfunction is not an inability to burn fat.
Comments
Post a Comment
Comment Moderation is ON ... I will NOT be routinely reviewing or publishing comments at this time..