A Fein(man), Fine Mess of Thermodynamics
I was truly baffled beyond all bafflement (is that a word?) that Feinman & Fine wrote "A calorie is a calorie" violates the second law of thermodynamics, let alone that it passed the peer review process to be published in any reputable journal. In the abstract they write:
Here, we propose that a misunderstanding of the second law accounts for the controversy about the role of macronutrient effect on weight loss and we review some aspects of elementary thermodynamics.
Here I propose that it is their apparent misunderstanding of thermodynamics, as put forth in this "study", that accounts for most of the gimmickry surrounding low carb diets in the community. In the section entitled "Thermodynamics", they write:
Hypothetically I could write any such reaction, but obviously not just anything I could make up will even be feasible, let alone happen. It must involve molecules that are realistic (not something that I'll bother covering here), but also the direction I choose to present doesn't mean anything. Thermodynamics tells us whether the reaction could proceed in that direction. I am going to use a reaction we know occurs, the combustion of methane (CH4 , main component of natural gas), as an example reaction: methane reacts with oxygen to form carbon dioxide and water.
The reactants are CH4 and O2 and the products are CO2 and H2O . The equation as written is unbalanced and incorrect because the numbers of each atom in the reactants and products are not the same. Above there are 1C, 4H and 2O in the reactants, and 1C, 2H and 3O in the products. We balance the reaction by playing with the proportions of the components:
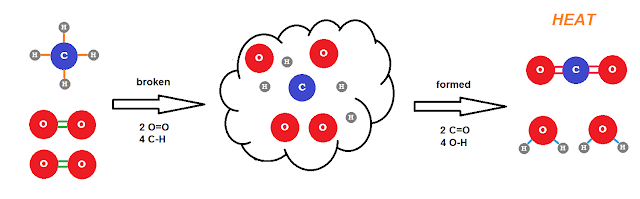
The above is oversimplified -- the cloud of isolated atoms in the middle is almost certainly NOT the mechanism by which this occurs -- but it's OK for the purposes of this post. In order to convert methane and oxygen to carbon dioxide and water, we must break four C-H bonds and two O=O double bonds, and then form two C=O double bonds and four O-H bonds. The energy to break/form bonds is referred to as enthalpy, H, and can be thought of as energy stored in the molecule. We can determine this stored enthalpy for the components of the chemical reaction and plot it vs. the "reaction coordinate" which is the progress of the reaction. Such a diagram looks like the one at right. Combustion is an example of an exothermic reaction. Essentially some of the internal enthalpy of the reactants is released to the surroundings in the form of thermal energy, heat. In a chemistry lab one will often be asked to feel the reaction vessel after mixing some chemicals, and if the tube warms this is evidence of an exothermic reaction. Reactions can also be endothermic which would look like the above diagram proceeding from right-to-left, or here.
Now, if what I'm about to present in any way contradicts what you've learned in a chemistry class, etc., please do go to your institution of higher learning or town tax authority and ask for your money back. This is basic stuff. It is not up for debate. We read chemical reactions from left-to-right:
The idea that "a calorie is a calorie" comes from a misunderstanding of the laws of thermodynamics. There are two laws of thermodynamics. (The zeroth law that establishes the concept of temperature and the third law that describes absolute zero are not relevant here). When speaking of "the laws of thermodynamics" it is important to be sure that one is including the second law. The first law is very different in character from the second law. The first law is a conservation law: it says that the form of energy may change, but the total is always conserved....
Reactants → Products
Hypothetically I could write any such reaction, but obviously not just anything I could make up will even be feasible, let alone happen. It must involve molecules that are realistic (not something that I'll bother covering here), but also the direction I choose to present doesn't mean anything. Thermodynamics tells us whether the reaction could proceed in that direction. I am going to use a reaction we know occurs, the combustion of methane (CH4 , main component of natural gas), as an example reaction: methane reacts with oxygen to form carbon dioxide and water.
CH4 + O2 → CO2 + H2O
The reactants are CH4 and O2 and the products are CO2 and H2O . The equation as written is unbalanced and incorrect because the numbers of each atom in the reactants and products are not the same. Above there are 1C, 4H and 2O in the reactants, and 1C, 2H and 3O in the products. We balance the reaction by playing with the proportions of the components:
CH4 + 2O2 → CO2 + 2H2O
The numbers in front of the O2 and H2O are like the coefficients of variables in an algebraic equation, so, for example, we have 2*2 = 4 O's on the left and 1*2 + 2*1 = 4 O's on the right. Since we are usually dealing with billions upon billions of atoms/molecules, we talk about a standard number of them called a mole instead of individual molecules: 1 mole = 6.02 x 1023 atoms or molecules. So the equation reads: 1 mole CH4 reacts with 2 moles O2 to produce 1 mole CO2 and 2 moles H2O. Stoichiometry is that strange word for this chemical math.
A chemical reaction is, on the most basic level, a rearranging the atoms from one molecular arrangement to another as crudely depicted below.
 |
| image link |
We use the delta, Δ, notation to denote "the change in" something: ΔX = Xafter - Xbefore . For a chemical reaction:
ΔHrxn = Hproducts - Hreactants
For the combustion of methane (source) ΔHrxn = -890 kJ/mol methane, so one way to think of this is to write the reaction as:
Note that energy is constant in an isolated system. Let's say we conduct the combustion in a vessel in an insulated enclosure of some sort (isolated system) and call everything besides the reaction "the rest":
CH4 + 2O2 → CO2 + 2H2O + 890 kJ Heat
Note that energy is constant in an isolated system. Let's say we conduct the combustion in a vessel in an insulated enclosure of some sort (isolated system) and call everything besides the reaction "the rest":
Htotal,isolated system = Hrxn + Hthe rest
ΔHtotal, isolated system = 0 ΔH the rest = - ΔHrxn
ΔHtotal, isolated system = 0 ΔH the rest = - ΔHrxn
So the reaction components lose 890 kJ energy when they react and rearrange, and this energy "lost" by the reaction is "gained" by "the rest" of the contents of the insulated enclosure as heat. It is important to understand that this evolution of heat in a chemical reaction is a perfectly acceptable application of The FIRST Law of Thermo, or as Feinman & Fine dismissively put it, that "bookkeeping law".
The second law is a dissipation law: it defines a quantity, the entropy, S, which we traditionally identify with disorder or high probability. The second law says that in any (real) irreversible process, the entropy must increase (ΔS > 0); balance is not expected. Entropy is, in fact, identifiable with irreversibility.
Before we go on, let's think about this. If this were true in the manner in which it is stated, then there would be NO real reversible processes! If A→ B is irreversible when ΔS>0, then we could never have B → A. Put another way, for any hypothetical A ↔ B, the ΔS is necessarily positive in one direction and negative in the other. Therefore, according to this statement by F&F, there can be no A ↔ B because the direction with +ΔS is irreversible. Put yet another way, in chemistry all reactions are theoretically reversible, those like combustion that we tend to think of as irreversible simply have highly favorable energetics in one direction and highly unfavorable (such as to be "impossible") in the other. They continue:
It is important to understand that it is the second law that drives chemical reactions. The first law is a bookkeeping law and tells us that the total energy attributed to work, heat and changes in chemical composition will be constant. It does not tell us whether such a reaction will occur, or if it does, what the relative distributions of the forms of energy will be.
I'll cut some slack on the semantics here -- "laws" don't drive reactions -- but they are trying to state that entropy drives all reactions and enthalpy is just along for the ride. False ... as in not true.
They are correct that thermodynamics only tells us the direction in which a reaction can proceed "spontaneously", but it tells us nothing about whether it will occur or the rate at which it will occur if it does. If we look back at the Energy, H vs. Reaction Coordinate diagram above, you see that the energy of the reactants initially increases and must overcome some threshold before the reaction moves "downhill" to the lower energy state products. This intermediate state is called the "activated complex" and the energy to attain that state is referred to as the activation energy, Ea. In combustion reactions, a match can typically provide this energy (heat evolved then provides it for subsequent reactions). Endothermic reactions tend not to be favored or occur spontaneously because, not only is energy required to push the reactants "uphill", but the activation energy will be even greater (in my diagram, Ea for the reverse = Ea as shown + ΔH, of as shown here). However not all exothermic reactions are favored either.
This statement, "[TFLOT] does not tell us ... what the relative distributions of the forms of energy will be" is misleading as well. While technically correct, TFLOT doesn't specify the forms of energy, it does predict 890 kJ of energy is liberated in the combustion of methane. True, we need to account for entropy losses (if any) to determine how much of that energy is available to do work (this is what Gibbs free energy is). But Feinman and Fine are confusing this issue with what our bodies -- through coordinated reactions -- do with the energy released. Tis true that a TFLOT application to our bodies -- the calories in/calories out energy balance -- cannot tell us whether the fatty acid is "burned" to fuel a muscle contraction or to generate heat to maintain body temperature. But let's be fair, TSLOT tells us diddly squat about that either, and if we eat protein vs. fat vs. carb, it tells us not how our bodies will use the energy. They continue:
They are correct that thermodynamics only tells us the direction in which a reaction can proceed "spontaneously", but it tells us nothing about whether it will occur or the rate at which it will occur if it does. If we look back at the Energy, H vs. Reaction Coordinate diagram above, you see that the energy of the reactants initially increases and must overcome some threshold before the reaction moves "downhill" to the lower energy state products. This intermediate state is called the "activated complex" and the energy to attain that state is referred to as the activation energy, Ea. In combustion reactions, a match can typically provide this energy (heat evolved then provides it for subsequent reactions). Endothermic reactions tend not to be favored or occur spontaneously because, not only is energy required to push the reactants "uphill", but the activation energy will be even greater (in my diagram, Ea for the reverse = Ea as shown + ΔH, of as shown here). However not all exothermic reactions are favored either.
This statement, "[TFLOT] does not tell us ... what the relative distributions of the forms of energy will be" is misleading as well. While technically correct, TFLOT doesn't specify the forms of energy, it does predict 890 kJ of energy is liberated in the combustion of methane. True, we need to account for entropy losses (if any) to determine how much of that energy is available to do work (this is what Gibbs free energy is). But Feinman and Fine are confusing this issue with what our bodies -- through coordinated reactions -- do with the energy released. Tis true that a TFLOT application to our bodies -- the calories in/calories out energy balance -- cannot tell us whether the fatty acid is "burned" to fuel a muscle contraction or to generate heat to maintain body temperature. But let's be fair, TSLOT tells us diddly squat about that either, and if we eat protein vs. fat vs. carb, it tells us not how our bodies will use the energy. They continue:
To predict the tendency of the reaction to occur, we must employ the second law that says the entropy must increase.
False. As in not true.
In a chemical reaction, at constant temperature and pressure, the entropic and energetic effects are combined into the change in the Gibbs free energy, ΔG, whose sign predicts the direction of reaction, and whose magnitude indicates the maximum amount of work realizable from the reaction.
In a chemical reaction, at constant temperature and pressure, the entropic and energetic effects are combined into the change in the Gibbs free energy, ΔG, whose sign predicts the direction of reaction, and whose magnitude indicates the maximum amount of work realizable from the reaction.
True. The Gibbs free energy of a system, G
G = H - TS
ΔGrxn = ΔHrxn - TΔSrxn
Or Goldfish are Hell with-out Tartar Sauce. The free energy of a reaction takes into account both the enthalpy, H, changes and the entropy, S, changes. So to say that only entropy drives chemical reactions is, again, flat out wrong. The spontaneous direction of a chemical reaction will be one where ΔGrxn < 0. When ΔGrxn = 0 the reaction is at equilibrium ... a topic for another day perhaps. In any case, let's look at the possibilities for what "drives" a reaction by making for a -ΔGrxn . If this is not discussed when one learns about Gibbs free energy, it should be! Realize, T in degrees K is always positive so the second term would be negative when ΔS is positive. We have four options for the signs of the enthalpy and entropy terms:
- -ΔHrxn (exothermic) and + ΔSrxn (increase entropy) → always -ΔGrxn
- +ΔHrxn (endothermic) and - ΔSrxn (decrease entropy) → always +ΔGrxn
- -ΔHrxn (exothermic) and - ΔSrxn (decrease entropy) → -ΔGrxn possible, favored at low T
- +ΔHrxn (endothermic) and + ΔSrxn (increase entropy) → -ΔGrxn possible, favored at high T
In option 1, the reaction is always spontaneous in the direction as written because mathematically you will always have ΔGrxn<0. Conversely, in option 2, the reaction cannot occur spontaneously in the direction as written, as mathematically you will always have ΔGrxn>0. Options 3 & 4 are a mixed bag of competing terms. A highly exothermic reaction will probably be spontaneous even if the change in entropy is not favorable, and since the entropy term is TΔSrxn>0, the impact of entropy losses will be smaller at lower temperatures. There would also be a temperature above which this term would dominate so where again you have ΔGrxn>0 and the reaction would no longer be spontaneous. To say that this situation in option 3 is TSLOT or entropy driving the reaction is clearly false. In option 3, enthalpy drives the reaction, entropy puts the brakes on. Lastly, in option 4, we have an endothermic reaction that can be driven by a highly favorable change in entropy. Since these reactions rely on a large negative TΔSrxn term in order for ΔGrxn<0, they are favored at higher temperatures.
Bottom line, both the energetic and entropic terms factor into whether a chemical reaction is spontaneous, as is the case for our combustion of methane example. All we need to do is start the ball rolling with a spark.
I think we can all agree that combustion is one of those quintessential "real, irreversible" reactions. And yet, this reaction is a #3 above, for combustion of methane (sources vary): ΔHrxn = -890 kJ/mol , ΔSrxn = -5.2 J/K-mol at 25°C = 298°K. Then:
ΔGrxn = -890,000 - (298)(-5.2) = -890,000 + 1550 = -888,450 = -888 kJ/molI think we can all agree that combustion is one of those quintessential "real, irreversible" reactions. And yet, this reaction is a #3 above, for combustion of methane (sources vary): ΔHrxn = -890 kJ/mol , ΔSrxn = -5.2 J/K-mol at 25°C = 298°K. Then:
The spontaneous, irreversible combustion of methane is highly driven by the energetics here, and although counterintuitive, the products of this combustion are in reality slightly more ordered than the reactants!
So the discussion of thermodynamics in this article, that is cited time and again, is wrong. I remember reading it some three years ago and just shaking my head. I recall pretty vividly learning for the first time about the equation for ΔG, and how the signs and magnitudes of both ΔH and ΔS determine what makes for a -ΔG. Thank you Mr. M. But ultimately, this is not the worst part of the article, it only sets the stage for more thermodynamic smoke and mirrors. Because, as I'm sure I've said before, the biggest BS is that after first mangling the laws of thermodynamics and going on about TSLOT, they then go on to make a TFLOT-based argument for the so-called metabolic advantage of low carbohydrate diets.
In Efficiency and Thermogenesis, they do some calculations employing the thermic effect of food, TEF. Using Jequier's factors, they replace carb calories with calories equally distributed between protein and fat to adjust "effective calories in". I have written about this before in Metabolic Advantage, Obesity and Eric Jequier. Thermogenesis in the human body from "burning" different molecules doesn't violate TFLOT or TSLOT any more than the thermogenesis of our methane combustion example. But this is a truly disingenuous argument to make as well. Because the supposed substitution that has fueled the obesity epidemic has been carb for fat when Americans en masse decided to follow the USDA Food Pyramid to a tee. If we did that replacement 1:1 between carb and fat, the high fat diet contains more effective calories vs. the high carb diet. Jonathan Bailor is even sneakier as he does the math for replacing carbs with protein -- as if somehow our collective lipophobia prompted us to replace protein in our diets with carbs. I've not seen any data showing American protein consumption has changed much if at all as a percent of calories.
But I think the reason that even protein doesn't seem to alter the effective calories in most studies I've read, is because it likely "comes out in the wash", to use a phrase. Here's why. For some reason, LCHF advocates treat thermogenesis as waste, but it's not! If we do not maintain a relatively constant body temperature -- that can differ dramatically (usually higher) from the environment around us even at comfortable temperatures -- we die. Our temperature is regulated, and for example, if we are placed in a cold environment, we ramp up so-called "futile cycles" to generate heat and/or uncoupling to divert energy to heat generation vs. ATP production. It is reasonable to presume that if we generate more heat digesting/metabolizing protein, we'll need less heat-generation elsewhere.
The major confusion Feinman and Fine are ultimately responsible for is that they liken our bodies to systems where thermal energy is harnessed to do mechanical work:
The efficiency of a machine is dependent on how the machine works and, for a biochemical machine, the nature of the fuel and the processes enlisted by the organism. A simple example is the inefficiency of low-test gasoline in high compression gasoline engines. If a "calorie is a calorie" were true, nobody would pay extra for high test gasoline. (The calorimeter values of a gasoline will be the same whether or not it contains an antiknock compound). In weight loss diets, of course, inefficiency is desirable and is tied to hormonal levels and enzyme activities.
Living beings do not operate that way. Warm blooded animals, such as us humans, "use" heat for ... well ... heat! Here is where individual variability will factor in. A body used to adequate nutrition may well put biological events into motion to "waste" energy from a large meal as heat. One that is chronically undernourished will probably not. But what ultimately rules here in the TFLOT context, is that more thermal energy is required to keep your body at 98.6 °F than at 97 °F, which is part of the basal metabolic rate: BMR/RMR/REE. It's not so much which chemical reactions we get the heat from ... but rather the temperature we maintain. I have to look for it, but I have a study in my Inbox that compared LC vs. LF diets and measured EE. The TEF for the LF diet was considerably greater, but if memory serves, total daily energy expenditures were similar, which would seem to support this idea.
 |
| image link |
To liken our bodies to car engines is misleading. First, because efficiency of combustion engines is something that requires the consideration of entropy. At right is a combustion engine. Fuel is ignited and the heat generated with combustion causes the gasses produced to expand thereby pushing the pistons down. This part of the process involves a conversion of thermal energy to mechanical work which can never be a complete conversion. The combustion engine is practically synonymous with TSLOT for most science and engineering majors, as it is probably the context in which most first learn about entropy and TSLOT. The key difference is that in such an engine we have:
Chemical Energy → Thermal Energy → Mechanical Energy
In our bodies we have: Chemical Energy → Thermal Energy and Chemical Energy → Chemical Energy and Chemical Energy → Mechanical Energy and Chemical Energy → Electrochemical Energy
The fuel selection for a combustion engine can improve efficiency by minimizing incomplete combustion, minimizing buildup of combustion products in the pistons, minimize friction in the piston, etc. I've yet to see any equivalence in a human body by altering the fuel composition in a body that is in energy balance. Further the fuel we burn -- as evidenced by the respiratory quotient, RQ, is almost never equal to the fuel we ingest, the food quotient, FQ -- because we convert fuels of one kind to another, and we don't even ingest significant amounts of ketone fuel. There's all this stuff about being a "fat burner" and obsession over the minutia of mitochondrial function in the community. It makes for interesting speculation, I suppose, but a goal of an inefficient metabolism -- where one can eat more -- seems counterproductive. We would desire a fuel mix that maximizes efficiency, but as Feinman and Fine stated, that's not really what we want for weight loss.
But one thing we can apply to the car engine and our human bodies: good old TFLOT. Because we can determine the caloric content of a gallon of fuel in a bomb calorimeter, and the ethanol-gasoline blends contain fewer calories per gallon than ordinary gasoline. The inherent inefficiencies of your engine are relatively independent of this so all that matters is that when you fill your tank with gasoline vs. an ethanol blend you can expect your car to go further on that tank. It's CICO for an inanimate object, put more calories in, get more calories out in terms of mechanical work. That TSLOT dictates that you don't get as many CO in mechanical work for the CI in fuel is irrelevant. Put more CI in the tank, get more CO in mechanical work. The calorie factors for protein, fat and carbs are averages and estimates. But they were determined for how the human body gets energy from food. The experiments the low carb hacks keep calling for to be done, already have been done. As Jules Hirsch explained in this Q&A with Gina Kolata:
Did you ever ask whether people respond differently to diets of different compositions?
Dr. Rudolph Leibel, now an obesity researcher at Columbia University, and I took people who were of normal weight and had them live in the hospital, where we diddled with the number of calories we fed them so we could keep their weights absolutely constant, which is no easy thing. This was done with liquid diets of exactly known calorie content.
We kept the number of calories constant, always giving them the amount that should keep them at precisely the same weight. But we wildly changed the proportions of fats and carbohydrates. Some had practically no carbohydrates, and some had practically no fat.
What happened? Did people unexpectedly gain or lose weight when they had the same amount of calories but in a diet of a different composition?
No. There was zero difference between high-fat and low-fat diets.
It truly disgusted me to listen to Jimmy Moore yucking it up on LCC waiting for Hirsch's old guard to *die off* so that we can finally convince people of this low carb crapola. Sheesh. Human metabolism may be less efficient in the face of drastic changes in one's diet as we may initially not digest/absorb nutrients as well, or spill ketones in urine. But we're pretty efficient at burning fuels once in the body. The molecules get many passes at the engines of our many cells, and excesses are stored until needed -- it's nothing like the one pass, one-way street in a combustion engine.
Bottom line, TEF is a term in TFLOT, plain and simple.
Lastly let's rewind to earlier in the article where Feinman and Fine "Apply ΔG". It's confusing what they're getting at, but they seem to contend that because Path 1 yields 4 cal/gram protein, and Path 2 yields 4 cal/gram carbohydrate, that Path 3 would have a ΔG of zero, but we know that 6ATP are required to convert one mole of alanine (amino acid) to glucose via the gluconeogenesis pathway. Somehow then,
... assuming that protein and carbohydrate are energetically equivalent leads to a contradiction.
Say what??? Let's say I could find two combustible fuels, where the hypothetical reactions with oxygen are: A + O2 → CO2 + H2O and B + O2 → CO2 + H2O And these two fuels, A & B, both yield the exact same 1000 kJ/mole A or B heat. Does that tell us anything about the reaction to convert A to B or vice versa? NO!! For starters, it may not even be mechanistically or energetically feasible to do the conversion. But for all we know it could take 5000 kJ to convert a mole of A to a mole of B, or it could take 5 kJ. This does not in any way impact the fact that via combustion each of these molecules release 1000 kJ energy in the form of heat. What's missing in the above figure, for one thing, is that there are other products besides those shown, as there would have to be for fuels A&B above.
To these guys, gluconeogenesis violates all laws of thermodynamics because no doubt the ΔG of this pathway is positive -- hence requiring ATP -- ATP that is a higher energy molecule that was created by electrochemically linking an energy producing reaction to the energy requiring reaction of ATP production from ADP. This is how our bodies work, folks. Most of the chemical reactions in our bodies are not energetically favored and/or would not occur spontaneously. The beloved coconut oil doesn't spontaneously form ketones in the jar. Indeed ATP is required to get the ball rolling to burn both glucose and fatty acids for energy. That we get, on average, 4 cal/gram from protein and carbohydrate does not in any way present some sort of disturbing contradiction requiring biochemical bamboozling. Speaking of pathways, however, most of the ATP produced when we metabolize carbs, fats or protein is produced in common pathways -- the exact same biochemical reactions, catalyzed by the exact same enzymes. The entropic losses in these pathways would be exactly the same. Then the ATP "fuels" everything else in the exact same way whether it was produced from a glucose molecule or a fatty acid or an amino acid.
Feinman has, in comments on Dr. Eades blog, made the case that gluconeogenesis accounts for the so-called metabolic advantage of low carb diets. This would, again, be a TFLOT argument. The energy required to produce glucose endogenously should be reflected in the metabolic rate, however, and Feinman routinely ignores how other energy-expensive processes such as de novo lipogenesis, would conversely be downregulated in the carb-restricted state.
All together, this is why, even when protein is varied considerably under controlled (in the sense of accountability, not the experimental sense) conditions, a calorie is basically a calorie.

Comments
"under controlled... conditions, a calorie is
basically a calorie."
Fine. Except that sentiment in practice (both medical practice and people's personal life) doesn't help much because nobody lives in controlled conditions. We are all subject to our "appetite" which is obviously a heavily neurohumerally driven process.
I suspect that this is the frustration that many people have with the "calorie is just a calorie" camp. On the surface it seems to ignore the likelihood that societal changes have occured that my have increased our appetite and led to the obesity epidemic. A few of my pet examples:
Diet sweeteners being heavily associated with weight gain, increased appetite, and affects on reward centers in the brain:
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2892765/
To me that opens the door to all the additives we put in our food to be biologically active. BPA's in our plastic, preservatives, etc. So maybe a calories is a calorie only when we don't eat frankenfoods?
Sleep duration and quality has decreased steadily over a similar time period that we have seen increased obesity. Then as people gain weight the risk of sleep apnea increases and the "obesity/sleep spiral" (my phrase) begins. Poor sleep increases ghrelin levels. I saw that in my own life after the birth of my second daughter three weeks ago. My appetite was crazy that first week I was home with that baby waking all night.
The basic fact is a calorie is a calorie, but that only controls weight not body composition not does it give a good indication of health. I'm not a health freak, LC, LF, or any other fad-diet person, just that people need to be aware of their nutritional needs outside of the basic calorie breakdown and be able to evaluate and change their diet as necessary to combat some basic health issues.
I also don't think your n=1 experiment with sleep is worth the time it took to write since postnatal your body is still normalizing and it's entirely possible that your body was signaling for more calories appropriately.
What's Worse: YoYo-Dieting or Constant Gluttony? What Happens During Weight Cycling? And Why Does Every Diet Make You Fatter? Lots of Questions, a Couple of Answers
I wouldn't say I'm giving hunger a "free pass" it's just a very, very, very basic need that people have a hard time denying. Like sex...ever heard how big an industry internet porn is? It's huge because people almost can't help themselves. I'm not giving porn surfers a free pass, just saying it's a tough impulse/addiction to fight.
Or better not to concentrate on Jimmy - pictures of different failed diet gurus and success stories, LC or whatever
Reproaching people for spending money on a t-shirt is cheap but not doing so for longer periods is worse :)
Further how many calories of protein are burned simply by digesting protein calories? What happens to free floating fatty acids in the body when insulin is not present?
I don't know how many times I hear an ignorant person talk about how lazy obese people are or how that just need to control their appetites. I often have to remind them if the fat was simply stored energy why are they not bouncing off of the walls?
Feinman and Fine erroneously equate thermogenesis with entropy losses. Eric reiterated a point I made in the post. If there's more TEF from protein v. fat or carb, our bodies just need to use less fat or carb to generate heat elsewhere.
When LC diets work for weight loss it is plain and simple because you eat fewer calories. The reason for this post was because this article was cited by Jonathan Bailor in his Scammier Pseudoscience of Slim book with two quotes from Feinman. Bailor again puts forth a thermodynamic argument for inefficiency of conversion of protein to body fat with thermogenesis as part of it.
Have you read Ned Kock's post on feeding time restriction? He touches on low carb toward the end: http://healthcorrelator.blogspot.com/2012/07/the-14-percent-advantage-of-eating.html
But I will never convince you or anyone else of that.
<snicker> when did we start weighing fatty liver as lean mass? </snicker>
Post a Comment
Comment Moderation is ON ... I will NOT be routinely reviewing or publishing comments at this time..