Remember LIRKO? Grey & Kipnis?
So a recent research paper has been making the rounds. It has a "red meat" title for the die hard TWICHOOB: Hyperinsulinemia Drives Diet-Induced Obesity Independently of Brain Insulin Production. This paper was brought up in my comments section as well. Every time this happens I really do feel like I'm in some sort of nutritional remake Groundhog Day. I'm a bit backlogged at the moment, but luckily Stephan Guyenet has weighed in with his analysis and included some additional studies in support of his position.
Whenever I think hyperinsulinemia causes obesity, I'm reminded of the LIRKO mouse. LIRKO is normal except its liver lacks insulin receptors. The result is a mouse with raging hyperglycemia and hyperinsulinemia throughout much of its young life until its liver poops out. I blogged on this mouse here: Bloggo Science ~ LIRKO Wars Edition. (Incidentally that post links to an older post by Stephan on the role of hyperinsulinemia here.) Here's the rundown on LIRKO:
- Hyperglycemic -- fasting and postprandial
- Hyperinsulinemic -- fasting and postprandial
- Normal glucose transport into muscle and adipocytes
- A pancreas that just doesn't quit
- Livers that suffer over time
- Normal weight
- Normal (slightly lower) intake
- Lower circulating NEFA
LIRKO should be a fat mouse by any version of TWICHOO. The hyperinsulinemia is caused by the hyperglycemia, that in this case is caused by unrestrained gluconeogenesis as the liver does not "see" insulin at all. This is a "simple" KO mouse to understand, and it should absolutely be a porker if TWICHOO had any validity. The latest TWICHOO X.0 hangs its hat pretty much unequivocally on IR causing obesity by inducing hyperinsulinemia. This is a rather considerable switch from postprandial insulin spikes leading to hyperinsulinemia but I can only work with what Taubes is claiming today. LIRKO is the test mouse for this version. Fail.
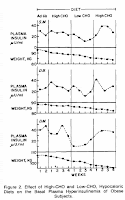

How about non-genetically modified humans? Well, it was a small study, but it was in a metabolic ward and the macros were varied quite widely. Grey & Kipnis. Here's a seminal blog post on this study as relates to Gary Taubes and the "good science" tome GCBC: GCBC Reference Check ~ Part IV of ? ~ Kipnis. Sure, these women were already obese, but if hyperinsulinemia caused fat accumulation or prevented fat breakdown, then this is your study. Incidentally it is among those dismissed by Taubes & Attia as not looking at a low enough carb diet. This is (a) in conflict with Taubes citing Kipnis in GCBC , and (b) not entirely correct as the weight loss phase involved essentially zero fat (sugar) diet vs. zero carb with steady weight loss despite wildly fluctuating insulin. Yeah, they didn't measure body composition, but do you really think there would be substantial differences on the iso-protein diets?
The mechanism by which hyperinsulinemia is purported to promote obesity is rather simple. Because of that, results in the LIRKO and studies like G&K are impossible to ignore. Folks like Peter/Hyperlipid make valiant attempts to explain them away making up ever more convoluted scenarios and misrepresenting facts, but in the end it comes back to this. In G&K we had women who were obese, thus clearly susceptible to insulin's evil actions on their fat cells. They failed to respond as TWICHOO claims. QED.
Comments
Looks like the 'new model' still has high-fat diet leading the way to obesity!
http://www.sciencedirect.com/science/article/pii/S1550413112001891
I don't think this is old enough to get from my usual source :(
To be fair, rodents tend to eat round the clock if you let them while humans normally do not.
I’m the serious scientist ☺ who wrote the paper some of you are discussing. I’m not a blogger (yet?), or a science writer, or someone’s struggling post-doc. I don’t favor any kind of freaky all-of-this or all-of-that-diet. I’m new to social media and I had never seen a nutrition or dieting blog before 48 hours ago. Wow, there is a whole universe of opinions out there (here)! Seriously, it is fantastic that so many people are thinking about metabolism etc.
Anyway, as a sort of experiment and learning process for myself, I decided I would follow my latest paper as it found its way into the ‘public sphere’. Many scientists are uncomfortable out in public, but I think it is important. So I decided, as a responsible scientist that I would not hide my ivory tower and I would help people understand the experiments if they were interested (rather than let other people speak on my behalf or just make stuff up). So, you can ask me on Twitter @JimJohnsonSci and I’ll do my best to answer your questions. I figure that if I can teach metabolism and physiology to a class of >700 3rd year students, the whole internet should be too hard, eh… (this may or may not be the dumbest thing I have done in a while).
I have found a few places, like this, where people are ‘discussing it’ but seem not to have a copy of it. Send me an e-mail and I’ll send you the paper. If you have any questions about the data, or our interpretation, you are welcome to ask me (instead of asking someone else who might not have read it or might have a particular agenda). I’m a basic scientist and I have no vested interest in any of the possible actions of insulin, or any of the other hormones or processes I study. I just want to find out how stuff works. In the current paper, we did a pretty straight forward experiment and got a pretty clear result.
I also can tell you what the paper does not address, and that includes different types of diets. We used a standard 58% high fat diet that is known to make mice (at least male mice) hyperinsulinemic. We have not yet tested other meal types, such as a high sugar diet. The paper was the culmination of 7 years and hundreds of thousands of dollars of work, so just doubling the number of experiments by adding another diet groups was not in the cards. I see no reason not to expect that any diet that chronically increases basal insulin, would show the insulin-dependent weight gain.
The press has some interest speculation about meal types, meal sizes and meal timing. There are some good data around these things, but our study does not directly address any of them.
Also, for the ‘mice-are-not-people’ crowd. Yes, I know. However, virtually all the studies on people can only come out with correlations, at best. Insulin and obesity have been correlated since the first radioimmunoassay was done decades ago. If you want to establish a causal role for a gene on a specific physiological function, you have to remove it and find out whether the physiology changes. Simple (in the lab). Rare, in the real world.
Cheers,
Jim
Anyway, insulin probably has multiple opposing effects on obesity, depending on the part of the body that it is acting in.
But first, THANKS for commenting here! I am truly honored that you have boldly ventured out into the blogosphere and shared your thoughts on my blog. I have the full text of your paper (actually the link in my post is to my sharing it in Google docs, if you'd rather I not do that please let me know) but haven't read it thoroughly, hence not weighed in yet as I try not to comment on research until I have an opportunity to do so. I promise to read it soon.
I was reminded of LIRKO, however, when I read this in your paper: "Moreover, knockout of the insulin receptor, by definition, induces tissue-specific insulin resistance, which alone may promote hyperglycemia and insulin hypersecretion (Bruning et al., 1998; Kitamura et al., 2003). Previous studies have been unable to determine the role of hyperinsulinemia on obesity in the absence of insulin resistance and glucose intolerance." That part is actually what prompted this post.
IMO, the description of LIRKO as the paper cited in my linked post ( http://www.sciencedirect.com/science/article/pii/S1097276505000158) is misleading. They say LIRKO has "dramatic insulin resistance, severe glucose intolerance". These mice are not IR or glucose intolerant when it comes to insulin action and/or glucose uptake in either muscle or adipose tissue. Their adipose tissue is insulin sensitive as demonstrated by their NEFA levels being normal if not a bit lower than normal. In the very next line they are unequivocal that "a failure of insulin to suppress hepatic glucose production and to regulate hepatic gene expression. These alterations are paralleled by marked hyperinsulinemia due to a combination of increased insulin secretion and decreased insulin clearance." To me this is not insulin resistance or glucose intolerance, it's excessive ENDOgenous glucose production that's the problem. And yet despite this, these mice are not obese. It would be interesting if they become more obese than normal mice on an HFD -- are you aware of anyone who has tested this?
Speaking of FIRKO, I think you might be interested in my posts on studies with ASP/receptor KO mice. This is the best link: http://carbsanity.blogspot.com/2011/10/fat-tissue-regulation-part-v-c5l2ko.html There are links to related KO mice. You are forewarned, that part of the fun I have here is to create cheezy Star Wars characters for KO mice using Lego character images. The thing is that ASP deficient and ASP fat receptor KO's mirror fat tissue in FIRKO and can make the ob/ob mouse obesity resistant.
Thank you for the interesting science and for your useful comment.
I linked to news of your paper in a comment here mostly because I knew it would be of interest to Evelyn's audience, but also in mild push-back to a particular humorous instance of her sometimes over-the-top bashing of a certain science writer. Despite often on-target critiques from our host, the writings of Taubes have had a strongly net-positive impact on the adiposity of certain English speakers - like me, and his current efforts to fund research are worthy of considerable praise.
An extremely interesting aspect of your paper involves those extra calories ingested by the Ins1+/-:Ins2-/- mice that were burned rather than stored. Perhaps your research will prove helpful in identifying diets and/or therapy that might promote a similar, even if less striking, response in humans.
As my responses got staggered, heck, let me welcome you again! I've been reading responses to your paper about the net and re-reading some of my own posts and your paper is of great interest to me. When I was doing the Fat Accumulation (linked to in comment below) series featuring KO mice I was looking for an insulin deficient one. It appears your mouse fills that gap! I'm hoping to be able to read it more carefully soon.
I see you've been welcomed to what can only be described as a dysfunctional community by the ever humble Dr. Eades' mockery. Sorry about that. On quick read, I think your conclusions may be over-reaching, but I hope that you do not think I'm belittling your research like Eades did.
Much of this community views studies such as yours through the lens of the carbohydrate-obesity hypothesis that used to go carbs → insulin spikes → chronic hyperinsulinemia → fat accumulation, but has now been modified to fructose → insulin resistance → hyperinsulinemia → fat accumulation. These ideas are the brainchild of science journalist Gary Taubes' resurrection of pre-war science and 60's endocrinology.
It was through critical analysis of his Good Calories Bad Calories book that I came across the works of Keith Frayn, Gunther Boden, Denis McGarry, Marc Hellerstein, and so many others I'll stop listing now for fear of leaving important names out. While I address personalities and such in this community on this blog, at least half of the posts over the going on three years have discussed peer review research specifically in the area of metabolism, more still on related topics. I was a research scientist in the pharmaceutical industry though I took a turn for my graduate work in materials science. I left the field years ago. In any case, I was researching the cause of some troubling symptoms I had developed on a low carb diet which led me to researching the role of free fatty acids in the development of type 2 diabetes, or NEFA, my preferred acronym. It's become more of a hobby, and occasionally obsession - grin, for me. The overwhelming bulk of the peer review literature in this area supports a mechanism that begins with excessive release of NEFA from adipose tissue.
In that regard, are you familiar with recent research Buettner's group on brain insulin resistance and adipose tissue lipolysis? http://www.jbc.org/content/287/39/33061.abstract It's something I've been meaning to blog on for a while now.
On the "man is not mouse" front, I'll have to await evidence there. In metabolic adaptation studies, rodents seem to have a far greater capacity either way (over/underfeed, restrict carbs, etc.) than humans. They can, and do when available, expend far more energy on maintaining body temp than we do, and most certainly have far more BAT activity than us. So I remain skeptical.
In any case, as you've discovered on Twitter the LC docs are at it mocking and doctoring your images to imply that your study supports their "carbs make you fat" theories. For my readers who don't follow me on Twitter, Eenfeldt altered the diagram to replace "high fat diet" with "bad food". I believe this is one reason for Stephan's response as well.
Anyway, the Asylum is also a haven for scientists seeking refuge from the scientist bashing that is so prevalent in this community. You're always welcome to the floor here. Having these discussions can only help increase everyone's understanding.
Regards,
Ev
“It appears your mouse fills that gap!”
Yes, this was the point. There are a lot of serious caveats with the IRKO approach. You can kind of pick our IRKO, but they have more issues than reported in the papers because insulin signalling (and IGF1 signalling) do more things than they knew when they wrote the papers. If one wants to know about what circulating insulin does ‘overall’, I think reducing insulin is the best way.
“I see you've been welcomed to what can only be described as a dysfunctional community by the ever humble Dr. Eades' mockery.”
Yes, amusing. I know lots of MDs and I’ve seen some arrogance in my time. Some of the best scientists are MDs, but they are never the ones you hear about. They are too busy doing their own research to write books. The smart ones know how little we know. Sadly, the minority.
“Much of this community views studies such as yours through the lens of the carbohydrate-obesity hypothesis“
From a biochemical and physiological standpoint, I think these are oversimplifications. As I have mentioned on Twitter a few times, I don’t think we should be focusing exclusively on the post-prandial state. In our data, it is the fasting insulin, not the stimulated insulin, that matches the obesity profile. The fasting insulin seems to be driven mostly by the proportion of beta-cell mass. This can be driven by fats, as well as carbohydrates. People should go an actually look up the diets we used before commenting.
“.. led me to researching the role of free fatty acids in the development of type 2 diabetes”
Yes, lipotoxicity. I have a whole program in my lab devoted to understanding its mechanisms in the islet and, what I think are interesting papers in PNAS, JBC, etc. We did the first unbiased proteomic analysis of lipotoxity in human islets (Jeffrey et al PNAS 2008)…. Most of my papers can by found on my google scholar page.
http://scholar.google.com/citations?user=7gAtalkAAAAJ&hl=en
“In that regard, are you familiar with recent research Buettner's group”
Of course, it is my job to be familiar with all the literature. Christophe saw my data pre-publication at a few conferences and we’ve chatted over dinner.
“On the "man is not mouse" front, I'll have to await evidence there”
These are minor issues. With regards to the core roles of insulin and its mechanisms, these are conserved from worms to man. There is plenty of evidence, so no need to await ☺ We need to see the forest from the trees.
“In any case, as you've discovered on Twitter the LC docs are at it mocking and doctoring your images to imply that your study supports their "carbs make you fat" theories. ... I believe this is one reason for Stephan's response as well. “
Yes, that is obnoxious. I bet none of them have read the paper… everyone wants a sound bite. As for Stephan’s response, it doesn’t seem like he really critiqued my paper, but rather found some old papers unlinking IR and obesity. We mention that these are unlinked, but I think insulin resistance (as variously defined) can be a red herring. I his comment about me not citing the literature was inappropriate and incorrect. In fact, I stashed 250 refs into the supplement. I’m known in scientific circles for being a rigorous citer. The data are the data. I still haven’t heard from anyone an alternate interpretation of the data we present in the paper. Also, bear in mind I have data from upcoming papers = extra information when making my interpretations.
“Anyway, the Asylum is also a haven for scientists”
The reason I chose your blog to reply to is that you had written a defense of scientists. However, I will probably switch mostly to twitter so I can keep the questions and answers short.
“It appears your mouse fills that gap!”
Yes, this was the point. There are a lot of serious caveats with the IRKO approach. You can kind of pick our IRKO, but they have more issues than reported in the papers because insulin signalling (and IGF1 signalling) do more things than they knew when they wrote the papers. If one wants to know about what circulating insulin does ‘overall’, I think reducing insulin is the best way.
“I see you've been welcomed to what can only be described as a dysfunctional community by the ever humble Dr. Eades' mockery.”
Yes, amusing. I know lots of MDs and I’ve seen some arrogance in my time. Some of the best scientists are MDs, but they are never the ones you hear about. They are too busy doing their own research to write books. The smart ones know how little we know. Sadly, the minority.
“Much of this community views studies such as yours through the lens of the carbohydrate-obesity hypothesis“
From a biochemical and physiological standpoint, I think these are oversimplifications. As I have mentioned on Twitter a few times, I don’t think we should be focusing exclusively on the post-prandial state. In our data, it is the fasting insulin, not the stimulated insulin, that matches the obesity profile. The fasting insulin seems to be driven mostly by the proportion of beta-cell mass. This can be driven by fats, as well as carbohydrates. People should go an actually look up the diets we used before commenting.
“.. led me to researching the role of free fatty acids in the development of type 2 diabetes”
Yes, lipotoxicity. I have a whole program in my lab devoted to understanding its mechanisms in the islet and, what I think are interesting papers in PNAS, JBC, etc. We did the first unbiased proteomic analysis of lipotoxity in human islets (Jeffrey et al PNAS 2008)…. Most of my papers can by found on my google scholar page.
http://scholar.google.com/citations?user=7gAtalkAAAAJ&hl=en
“In that regard, are you familiar with recent research Buettner's group”
Of course, it is my job to be familiar with all the literature. Christophe saw my data pre-publication at a few conferences and we’ve chatted over dinner.
These are minor issues. With regards to the core roles of insulin and its mechanisms, these are conserved from worms to man. There is plenty of evidence, so no need to await ☺ We need to see the forest from the trees.
“In any case, as you've discovered on Twitter the LC docs are at it mocking and doctoring your images to imply that your study supports their "carbs make you fat" theories. ... I believe this is one reason for Stephan's response as well. “
Yes, that is obnoxious. I bet none of them have read the paper… everyone wants a sound bite. As for Stephan’s response, it doesn’t seem like he really critiqued my paper, but rather found some old papers unlinking IR and obesity. We mention that these are unlinked, but I think insulin resistance (as variously defined) can be a red herring. I his comment about me not citing the literature was inappropriate and incorrect. In fact, I stashed 250 refs into the supplement. I’m known in scientific circles for being a rigorous citer. The data are the data. I still haven’t heard from anyone an alternate interpretation of the data we present in the paper. Also, bear in mind I have data from upcoming papers = extra information when making my interpretations.
“Anyway, the Asylum is also a haven for scientists”
The reason I chose your blog to reply to is that you had written a defense of scientists. However, I will probably switch mostly to twitter so I can keep the questions and answers short.
Before I forget, I'd love the supplement with the references you referred to, as well as the timed-feeding study. Carbsane at gmail dot com, but I'll also try and email you when I get a chance. For some reason gmail is not working and playing well with this browser on this computer at the moment :(
In any case, I'm probably more "tainted" against mouse models of adiposity and disease lately because several months back I started looking into the GLUT4 KO mice. Some VERY interesting stuff there, all about 5 or so years old and implicating RBP. Well, turns out we don't see that in humans which is likely why we're not still seeing all of the unanswered questions answered in intervening years. Still, some basics hold between mice and humans.
So yeah, insulin has similar actions, but some of the processes it is involved in are more significant in mice than humans. (The role of de novo lipogenesis and gut-derived SCFA come to mind as well). There's also no getting around that small rodents expend a lot more energy as a percent of overall metabolism on body temperature regulation and adaptations seem to be available within a far greater range than for humans. If there are studies in humans where suppressing insulin is shown to upregulate WAT UCP1 (with octreotide perhaps?), I'd be very interested.
If I may, it seems (again, I plan to devote appropriate attention to your paper later this week or over the weekend) that in your mouse, failure to be able to compensate with insulin induces an adaptive pathway that "burns off" the lipids. This produces healthy mice, apparently. But we do not see this in humans -- or is there even an analogous condition?
To me this illustrates the beauty of the KO, and the limitations. It can isolate an action with pinpoint accuracy and help unmask others that may kick into the forefront. But unless there's an analogous genetic defect in humans, they are of little practical application. On the flipside, however, they can help elucidate the difference between mechanism of pathology vs. symptomatic agent. For example, if hyperglycemia alone causes problems, this mouse should be blind and limbless by 6 mo. If insulin alone regulates fat mass, it should be a porker. So KO's provide better information than trying to infuse an animal etc.
I don't think any serious person denies the role insulin plays in the PROPER storage of fat in adipose tissue.
My gut says your mouse demonstrates well that the body must be capable of high basal insulin production in order for obesity to develop and be maintained. But what causes that high basal insulin? I think there's ample evidence that it is compensatory to overnutrition and expanding fat stores in non-gen-mod animals and humans.
Interestingly enough, if HI comes before IR, the LC intelligentsia just shot their science journo leader in the foot ;-) But I doubt you wanna get entangled in that web!
Cheers!
I don't expect you will be reading this, but if you are, I have a question. Have you looked at the pattern of insulin secretion in your mice? You will know all about insulin oscillations and how important they are for insulin action. I have seen a suggestion in the literature that a high fat diet disrupts oscillatory insulin secretion. Could it be more disrupted in the Ins+/+ mice than in the Ins+/- mice?
You told Evelyn earlier that 'the mice that are fed only in a small window of time are protected from the hyperinsulinemia'. This could mean, mice that do fasting-induced autophagy have well-functioning beta cells which oscillate properly. I'm sure this has occurred to you.
This misconception makes a lot of studies about high fat diet a bit problematic for extrapolating the results in our current diets.
Do not know if this is the case of your study (sorry if not) but just want to note it.
Post a Comment
Comment Moderation is ON ... I will NOT be routinely reviewing or publishing comments at this time..