The BE&HM Series ~ Part VII: Chemical Reactions
It's been a while here, and I'd really rather move forward on some other topics, but I had most of this already in the "hopper" and wish to refer to it in a coming post on calories, so a couple of shorties here. Also since it's been a while, here are links to the other posts in this series (in order): Biophysical Electrochemistry and Human Metabolism, Part II: Atoms & The Periodic Table, Part III: The Main Group Elements, The Octet Rule and Ions, Part IV: Ionic Compounds, Elements & Oxidation State Defined, Part V: Covalent Bonding & Molecules, Part VI: Electron "Ownership" & Polarity.
Most of this is copied from another post here at the Asylum, so if it seems familiar to any regular readers, that's why. I'm going to be using the combustion of methane as an example in the next post on oxidation, so I might as well use it as an example here.
Reactants → Products
Thermodynamics tells us whether the reaction can proceed in that direction (but not if it will or the rate at which it proceeds). Methane reacts with oxygen to form carbon dioxide and water.
CH4 + O2 → CO2 + H2O
The reactants are CH4 and O2 and the products are CO2 and H2O. The equation as written is unbalanced and incorrect because the numbers of each atom in the reactants and products are not the same. Above there are 1C, 4H and 2O in the reactants, and 1C, 2H and 3O in the products. We balance the reaction by playing with the proportions of the components:
CH4 + 2O2 → CO2 + 2H2O
The numbers in front of the O2 and H2O are like the coefficients of variables in an algebraic equation, so, for example, we have 2*2 = 4 O's on the left and 1*2 + 2*1 = 4 O's on the right. Since we are usually dealing with billions upon billions of atoms/molecules, we talk about a standard number of them called a mole instead of individual molecules: 1 mole = 6.02 x 1023 atoms or molecules. So the equation reads:
1 mole CH4 reacts with 2 moles O2 to produce 1 mole CO2 and 2 moles H2O
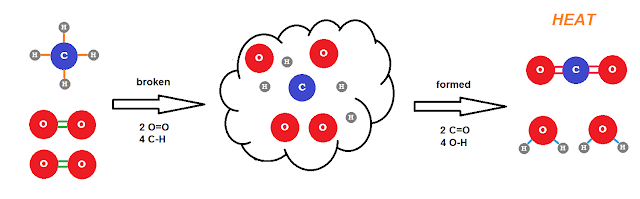
Stoichiometry is that strange word for this chemical math. A chemical reaction is, on the most basic level, a rearranging the atoms from one molecular arrangement to another as crudely depicted below.
 |
| image link |
We use the delta, Δ, notation to denote "the change in" something: ΔX = Xafter - Xbefore . For a chemical reaction:
ΔHrxn = Hproducts - Hreactants
For the combustion of methane (source) ΔHrxn = -890 kJ/mol methane, so one way to think of this is to write the reaction as:
Note that energy is constant in a closed system. If we are able to contain combustion in a thermally insulated vessel of some sort that we shall call the surroundings:
CH4 + 2O2 → CO2 + 2H2O + 890 kJ Heat
Note that energy is constant in a closed system. If we are able to contain combustion in a thermally insulated vessel of some sort that we shall call the surroundings:
Htotal,closed system = Hrxn + Hsurr ΔHtotal, closed system = 0 ΔHsurr = - ΔHrxn

So the reaction components lose 890 kJ energy when they react and rearrange, and this energy "lost" by the reaction is "gained" by the surroundings as heat.
Before moving on, note that there is a threshold energy, Ea = activation energy, that must be exceeded before the reaction proceeds. This is a very generic depiction of reaction energetics and the implication that there is a relationship between the magnitudes of ΔH and Ea is misleading in the diagram. You can have reactions with small Ea and large ΔH, and we can have reactions with large Ea and small ΔH, both small, both large, you get the picture. The reactants "transform" to what is called the "activated complex" or "transition state" at the peak of the curve and then proceed downhill. One can draw the analogy to a car at some higher elevation than another, it will roll downhill given a path to do so. If it's on the other side of a hill, it must make it to the top of the hill requiring gas to get there, but once over the top, you can shut off the engine and coast down to the bottom.
Reactions can also be endothermic. Both are shown below:

In this direction, there's a greater "hump" to overcome as well as energy being "required" . But all chemical reactions require overcoming a threshold before the reaction proceeds spontaneously. If this were not so there would be no stable molecules.
Enthalpy is not the only energetic term in consideration, or otherwise only exothermic reactions would likely ever occcur! The whole picture includes an entropy term, ΔS that combines with enthalpy to determine "free" energy ΔG: G = H - TS or ΔGrxn = ΔHrxn - TΔSrxn
Biochemistry Note: I may revisit this and flesh out the full energetics, or address it elsewhere at some point. It is my position that we don't really need to bother with the entropy in the types of discussions I'm about to enter into, and here's why:
- The major energy "currency" of the human body is adenosine triphosphate, ATP
- The vast majority of ATP production from the breakdown of all three macronutrients is from the point of acetyl CoA onward (Krebs/TCA and the ETC) through common pathways, and
- ATP subsequently fuels energy requiring processes.
In these processes, we have interconversion of chemical energies. There are many who are generally first (and perhaps only) exposed to thermodynamics in the physics/MechE context of the steam engine/Carnot cycle. In that context, the heat energy released is converted to mechanical work -- a conversion that is never 100% efficient -- this loss is chalked up to entropy. This is not a knock on those to whom I refer, it is merely a statement of fact. In the ATP (and related) coupled reactions scenarios, we don't have these losses because humans do NOT work by trying to convert thermal energy to mechanical energy like steam engines.
Comments
Sounds like you guys are similar.
The Caloric content of our food is really nothing more than a convenient convention with little basis in reality. eg PUFA and MCTs are metabolised in a totally different manner despite having an identical caloric value.
of writing, in my view its genuinely amazing designed for me.
My homepage :: diets that work fast for women
web page, it includes priceless Information.
Also visit my weblog - subzero refrigerator & appliance repair Safety Harbor FL
Feel free to visit my blog post: washer and dryer appliance repair Dunedin FL
again to read other news.
Feel free to visit my web blog; www.Amityspace.com/blog/96923/Mature-Chat-sites-may-Be-entertaining/
revisiting. I wonder how a lot effort you set to create one of these excellent informative website.
Also visit my web site :: Appliance Repair Oldsmar
Feel free to surf to my page: zubzero refrigerator Lutz (http:
//www.youtube.com/)
Post a Comment
Comment Moderation is ON ... I will NOT be routinely reviewing or publishing comments at this time..