The BE&HM Series ~ Part VIII: Electron "Ownership" & Non-Reversible Oxidation
Previous posts in this series: Biophysical Electrochemistry and Human Metabolism, Part II: Atoms & The Periodic Table, Part III: The Main Group Elements, The Octet Rule and Ions, Part IV: Ionic Compounds, Elements & Oxidation State Defined, Part V: Covalent Bonding & Molecules, Part VI: Electron "Ownership" & Polarity, Part VII: Chemical Reactions
In a lot of the discussions of mitochondria and the various metabolic pathways in general, the term "redox" gets thrown around a lot. Redox is a contraction of the terms reduction and oxidation. This generally applies to reversible reactions where one species is oxidized and the other reduced, but it actually applies to all reactions wherein electrons are "transferred" from one element to another. Where I'm going here (and I don't know when I'll get there, but I will) is to discuss redox couples and basically how we are electrochemical machines where one chemical reaction is coupled with another -- the energy released from the favorable reaction drives the otherwise unfavorable chemical reaction to which it is coupled.
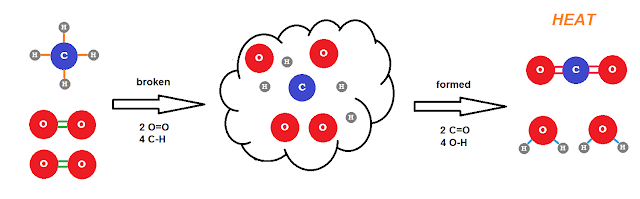
For our irreversible oxidation reaction, I'll again use the combustion of methane as an example:
In a lot of the discussions of mitochondria and the various metabolic pathways in general, the term "redox" gets thrown around a lot. Redox is a contraction of the terms reduction and oxidation. This generally applies to reversible reactions where one species is oxidized and the other reduced, but it actually applies to all reactions wherein electrons are "transferred" from one element to another. Where I'm going here (and I don't know when I'll get there, but I will) is to discuss redox couples and basically how we are electrochemical machines where one chemical reaction is coupled with another -- the energy released from the favorable reaction drives the otherwise unfavorable chemical reaction to which it is coupled.
For our irreversible oxidation reaction, I'll again use the combustion of methane as an example:
CH4 + 2O2 → CO2 + 2H2O

In the installment about polarity, I talked about a concept I called "electron ownership". Some atoms have more electron affinity or drawing power or ability to hold onto electrons than others. Oxygen is one such atom, whereas hydrogen, with it's single proton, is virtually helpless when it comes to holding on to its single electron. In their ground (elemental, pure, only that atom) state, each atom starts out with the same number of electrons as it has protons. When an atom takes on more or loses electrons fully, it becomes an ion -- in a way, the ultimate oxidized (or reduced) state. An atom "owning" fewer than its ground state e's is said to be oxidized, and one "owning" more is said to be reduced. See here for where I first discussed this in this series. In covalent compounds, however, there is unequal sharing of electrons but not complete transfer. The oxidation state, or oxidation number, associated with an atom indicates the "effective" charge by virtue of the number of electrons it is either credited with in excess of ground state, or missing.
For the relevant bonds here:
C-H: the electrons go to C
O=O: ground state
O-H: the electrons go to O
C=O: the electrons go to O.
So in methane, the C gets 1e from each H and has an ox# of -4 and O has an ox# 0 in oxygen gas. After combustion, the O has 1e from each H in water and has an ox# -2 and each gets 2 from the C in carbon dioxide also having an ox# of -2 leaving C with an ox# of +4. H has an ox# of +1 throughout the reaction. Thus for the oxidation numbers we have:
C: -4 to +4 (oxidized) O: 0 to -2 (reduced) H: +1 (no change)
Taking into account that we have 2O2 consumed and 2H2O produced, there are a total of 8 e's "transferred" from one C to 4O (2 e's each). We can break the reaction down into two half reactions:
Where this series is headed ...
In the next installment, I am going to discuss redox reactions in a context in which they are more commonly introduced in chemistry: electrochemistry of metals (inorganic). This is basically the last piece in the puzzle -- though it may require more than one post -- before I can put it all together and we can look at biochemistry as the orchestrated electrochemical dance that it is.
Integral to this will be how chemical energy is seemlessly transformed from one type to another without the need to be converted to thermal energy, aka heat. This chemical energy is calories, or Calories, or kcals, or Joules, or ergs, or even foot pounds, though that unit would be out of place in this realm. In biological systems, reactions are coupled such that the energy from one favorable reaction is used to drive others, and us warm blooded creatures use the heat that may "escape" as part of the regulated grand scheme of things to keep warm. On occasion we even "waste" some of the chemical energy destined for "work" to stay warm.
So let's look back at the combustion of methane. One mole (that is 6.02 x 10^23 molecules) of methane, reacted with two moles of O2 gas releases 890 kJ of heat (source). If I do this on the burner of my gas stove, and sit a kettle of water on the flame, there are apparently some that would argue that my water never gets warm, let alone boils, because I need calories for this purpose. After all, a calorie is defined as the amount of (thermal) energy required to raise 1g of water 1°C and the Joules from the combustion of methane just won't do the trick. This is, of course, an absolutely ridiculous statement to make: that 890 kJ of heat energy is in every way the same as 212.7 kcal of heat energy ... and it came from the chemical energy stored in the reactants.
What happens to that energy? Well, some will escape into the environment (heat the air), some will heat the kettle, and the rest will heat the water at 1cal heating 1g of water 1°C. Water boils -- changes state to gas -- at 100°C (standard atmospheric pressure). So if there is sufficient heat energy created from the combustion of methane to heat the water to this temperature, the additional energy will now be consumed in vaporizing the water, to the tune of 2.26 kJ/g (source) ... or calories or ergs or whatever energy unit you're working with.
In my example, we are essentially "coupling" the combustion reaction with the water when we place the kettle on the flame. The heat generated in the chemical reaction is transferred to the water, and depending on the situation either warms it (increases thermal energy content of the water) or causes it to undergo the phase change from liquid to gas or both (provides energy for phase change). If instead we perform the same combustion in a piston, the combustion heats the gas causing it to expand, pushing the piston up and this motion can be translated, for example, into forward motion of a vehicle. In this context, we are harnessing thermal energy and converting it into mechanical "work" energy. A very simplistic statement of the Second Law of Thermodynamics is that the conversion of Heat → Mechanical Energy is NEVER 100%. You can never convert 1 J or 1 cal or 1 erg of heat to 1 J or 1 cal or 1 erg of mechanical work. This is the part about thermal energy that involves entropy and TSLOT. But this is also something we need not worry over because our bodies do not attempt to convert thermal energy to other forms. As I've said many times: Heat generation is a perfectly acceptable "out" term for the FIRST Law of Thermo.
Here's where I'm ultimately going...
The picture at right is of the "Fatty Acid Spiral" -- the β-oxidation of the common saturated fatty acid, 16C palmitic acid. Do you see any heat anywhere? No*. What you have is (1) remaining chemical potential energy in the 7 Acetyl CoA moieties that are headed for Krebs (where ATP and more NADH & FADH2 is produced), and (2) "Reducing equivalents" in the form of NADH & FADH2. Then it's off to the Electron Transport Chain for all of the NADH and FADH2 to generate the bulk of the ATP.
Where are the calories? Still in chemical potential energy at this point. We've transferred some from the long chain fatty acid bonds to the AD's, and there's some remaining in the Acetyl-CoA. We have oxidized the long chain fatty acid. The carbon atoms have lost ownership of the electrons, just like our methane. Electrons and energy has changed hands if you will. We don't have CO2 yet and we don't have heat*. Stay tuned...
(*there may be some heat production at this stage, but the majority of the energy released in breaking down the fatty acid is transferred to other chemicals, any heat generated basically goes towards keeping you warm.)
Oxidation: C(-4) → C(+4) + 8e
Reduction: 4O(0) + 8e → 4O(-2)However we rarely refer to this as a redox reaction mostly because it's the oxidation -- "burning" -- of the methane that is of importance. The energetics of this reaction are such that it is, for all intents and purposes, irreversible. The highly exothermic reaction would require too much energy to reverse. (By contrast, the combustion of hydrogen: 2H2 + O2 → 2H2O is highly favored, but not so energetically "imbalanced" that we can't make it go the other way pretty easily. See electrolysis of water.)
Where this series is headed ...
In the next installment, I am going to discuss redox reactions in a context in which they are more commonly introduced in chemistry: electrochemistry of metals (inorganic). This is basically the last piece in the puzzle -- though it may require more than one post -- before I can put it all together and we can look at biochemistry as the orchestrated electrochemical dance that it is.
Integral to this will be how chemical energy is seemlessly transformed from one type to another without the need to be converted to thermal energy, aka heat. This chemical energy is calories, or Calories, or kcals, or Joules, or ergs, or even foot pounds, though that unit would be out of place in this realm. In biological systems, reactions are coupled such that the energy from one favorable reaction is used to drive others, and us warm blooded creatures use the heat that may "escape" as part of the regulated grand scheme of things to keep warm. On occasion we even "waste" some of the chemical energy destined for "work" to stay warm.
So let's look back at the combustion of methane. One mole (that is 6.02 x 10^23 molecules) of methane, reacted with two moles of O2 gas releases 890 kJ of heat (source). If I do this on the burner of my gas stove, and sit a kettle of water on the flame, there are apparently some that would argue that my water never gets warm, let alone boils, because I need calories for this purpose. After all, a calorie is defined as the amount of (thermal) energy required to raise 1g of water 1°C and the Joules from the combustion of methane just won't do the trick. This is, of course, an absolutely ridiculous statement to make: that 890 kJ of heat energy is in every way the same as 212.7 kcal of heat energy ... and it came from the chemical energy stored in the reactants.
What happens to that energy? Well, some will escape into the environment (heat the air), some will heat the kettle, and the rest will heat the water at 1cal heating 1g of water 1°C. Water boils -- changes state to gas -- at 100°C (standard atmospheric pressure). So if there is sufficient heat energy created from the combustion of methane to heat the water to this temperature, the additional energy will now be consumed in vaporizing the water, to the tune of 2.26 kJ/g (source) ... or calories or ergs or whatever energy unit you're working with.
In my example, we are essentially "coupling" the combustion reaction with the water when we place the kettle on the flame. The heat generated in the chemical reaction is transferred to the water, and depending on the situation either warms it (increases thermal energy content of the water) or causes it to undergo the phase change from liquid to gas or both (provides energy for phase change). If instead we perform the same combustion in a piston, the combustion heats the gas causing it to expand, pushing the piston up and this motion can be translated, for example, into forward motion of a vehicle. In this context, we are harnessing thermal energy and converting it into mechanical "work" energy. A very simplistic statement of the Second Law of Thermodynamics is that the conversion of Heat → Mechanical Energy is NEVER 100%. You can never convert 1 J or 1 cal or 1 erg of heat to 1 J or 1 cal or 1 erg of mechanical work. This is the part about thermal energy that involves entropy and TSLOT. But this is also something we need not worry over because our bodies do not attempt to convert thermal energy to other forms. As I've said many times: Heat generation is a perfectly acceptable "out" term for the FIRST Law of Thermo.
Here's where I'm ultimately going...
 |
| image link |
Where are the calories? Still in chemical potential energy at this point. We've transferred some from the long chain fatty acid bonds to the AD's, and there's some remaining in the Acetyl-CoA. We have oxidized the long chain fatty acid. The carbon atoms have lost ownership of the electrons, just like our methane. Electrons and energy has changed hands if you will. We don't have CO2 yet and we don't have heat*. Stay tuned...
(*there may be some heat production at this stage, but the majority of the energy released in breaking down the fatty acid is transferred to other chemicals, any heat generated basically goes towards keeping you warm.)
Comments
Post a Comment
Comment Moderation is ON ... I will NOT be routinely reviewing or publishing comments at this time..