The Second Law of Thermodynamics in Humans: For Heat's Sake!
 On the urgings of a few commenters here on the blog, and even more recently, by Don't-Call-Him-John (Kiefer), I've found myself reading Biological Thermodynamics by Donald Haynie (2nd edition). [It's #1 on this Amazon Search
On the urgings of a few commenters here on the blog, and even more recently, by Don't-Call-Him-John (Kiefer), I've found myself reading Biological Thermodynamics by Donald Haynie (2nd edition). [It's #1 on this Amazon Search
After reading large chunks of the book -- had a lot of time on my hands without internet access these past couple of weeks -- it has become even more clear as to where some of the biggest misconceptions about thermodynamics in this community come from. It is not that Haynie is incorrect, however. There are many statements in the book that read as "off", but on second or third pass through are technically accurate provided one is using his definitions in proper context*. It is more that his book is so filled with examples that lack relevance to biological contexts as to cloud the picture. What results is that although somewhere in the text all the relevant "prerequisites" are stated, the final statements almost beg to be taken out of context!!
It is ironic, to me, that the First Law always applies. It applies to open, closed and isolated systems provided all energy terms are accounted for. And yet many in this community think it does not apply to open systems, rather they believe it is the Second Law that governs open systems. In reality, when looking at just the system, the Second Law is the one that relates exclusively to isolated systems. It's even right there on Wikipedia! An increase in entropy is indeed the only "driving force" for changes in the distribution of energy in an isolated system (one which exchanges neither energy nor matter with its surroundings). And yet decreases in entropy for, say, the components of a reaction, occur all the time. For example, the entropy change of a system consisting of methane and oxygen that is ignited, is negative:
CH4 + 2O2 → CO2 + 2H2O ΔSsystem= -7.10 J/K
This is because the entropy of the surroundings is increased and thus the Second Law holds, but this is but one of many examples of how the Second Law misstated (or applied out of context) has led to rampant confusion.
In Chapter 3 on The Second Law of Thermodynamics, Haynie includes a section on heat engines. Here's a screen shot of my annotated PDF (how much do I love thee Adobe Acrobat XI ... I cannot count the ways!)
In this section he goes on to describe "waste heat" - qwaste - as heat that cannot do work. I have gone over this many times here in the past. I cannot stress enough that what is written in Haynie's book is in perfect agreement with what I have been saying. A steam, heat or combustion engine all rely on the expansion of gasses when heated to do mechanical work -- e.g. push a piston outward from the cylinder base. This is referred to as PV work in both the book and in the context of the gas laws (PV=nRT anyone?). The problem with this is that NO work of this nature, ever, is done in the human body. It is NOT how our "engines" operate. Which is what Haynie tells his readers off the bat. So the problem here is not in Haynie's description or definition of waste heat, it is the context.
(Yet) Another Analogy ...
Here in the Northeast, many of us heat our homes with oil. Heating oil is combusted in the furnace for the sole purpose of generating heat, which is eventually transferred to the air in the home. Would anyone consider this heat to be waste? Of course not!
What if, instead, my furnace was a duel purpose combustor? What if I not only used it to heat my home, but also as an electricity generator. In fact, what if most of the time I used it to generate electricity and heat was a secondary use. Would heat then be a waste? Not necessarily, but it could be in certain contexts. For example if I could only run my furnace to generate heat and electricity at all times, then in the summertime, the heat would most certainly be waste. If I could program my furnace to only generate electricity, then heat would never be waste. Unfortunately, I could not run my furnace without generating some heat, therefore if it's not useful, heat is a waste product in any system used to convert chemical energy to another form.
Our human biochemical furnaces are like my duel purpose furnace. The primary purpose is to produce ATP to fuel all the other work that needs to be done, with the necessary and inescapable production of some heat at virtually every step along the way. This was an additional point made in my last post on this topic: Mechanical Work, ATP, and Macronutrients (and Thermodynamics). Some, like Christopher who challenged me in comments there, might be interested in this quote from Haynie (p. 5)
A deeper sense of the nature of energy flow can be gained from a bird’s-eye view of the biological roles of adenosine triphosphate (ATP), the small organic compound that is known as “the energy currency of the cell.” This molecule is synthesized from solar energy in outdoor plants and chemical energy in animals. The detailed mechanisms involved in the energy conversion processes are complex and extremely interesting, but they do not concern us here. The important point is that once it has been synthesized, ATP plays the role of the main energy “currency” of biochemical processes in all known organisms. ATP provides the chemical energy needed to “power” a huge variety of biochemical process, for example, muscle contraction. ATP is involved in the synthesis of deoxyribonucleic acid (DNA), the molecular means of storing and transmitting genetic information between successive generations of bacteria, nematodes, and humans. ATP is also a key player in the chemical communications between and within cells. ATP is of basic and central importance to life as we know it (Fig. 1.6).
As well as this one (p. 41)
Combustion of food in a bomb calorimeter tells us more than just how much heat is produced when food is completely burnt to a crisp. Indeed, tables of oxidation would be of little use to nutritionists if the numbers did not say something about the energetics of metabolism. Such tables are useful to the physicist, the biochemist, and the nutritionist because the laws of physics are assumed to be independent of time and location. In other words, the enthalpy of oxidation of glucose is not one value in a bomb calorimeter and some other value in the striated muscle connecting your big toe to the rest of your body. By Hess’s Law, this enthalpy equivalence holds despite the fact glucose oxidation occurs in the body by a large number of sequential steps involving a large number of chemical intermediates. This discussion suggests that we can use machines like calorimeters to investigate the thermodynamic properties of the body and the molecules the body is made of. It also suggests that our bodies themselves are very much like machines.
This second quote is essentially the First Law. If we ingest and absorb glucose, and eventually excrete CO2 and H2O, the calories of energy we have derived from that glucose are the same as those we measure in a calorimeter. Furthermore, from the point of ATP forward, we are at "calories out" -- in other words, using that energy "gained" to do stuff, aka "work". This is the same ATP no matter where the energy came from to produce it.
The pathways of energy production converge at acetyl-CoA, the major entry point into the Krebs (TCA) Cycle. These are specific, regulated and orchestrated chemical reaction pathways driven by "biological manipulation" such that there is a net negative change in free energy when reactions are coupled. What happens to the excess? Heat. Free energy is, after all, that which is AVAILABLE to do work, but it doesn't mean it has to do work. If you burn gasoline in a fire pit, lots of free energy is released, it just doesn't do any work unless the combustion is contained in such a way as to do so. So we produce heat as a byproduct of metabolism at pretty much every turn. Indeed the thermogenic factors of the macros are a reflection of how much heat is evolved compared with ATP produced.
But harkening back to my duel purpose furnace. If I lived on Antarctica the heat produced by this furnace would never be waste to my system (house). This is the situation for warm blooded animals such as humans. Our operating temperatures are within a range of only a few degrees, and even a mild 75 degree day might as well be Antarctica to our individual cells. The smaller an animal, the greater it's surface area to volume or mass ratio, the more it dissipates (transfers, loses) heat to the environment -- the more such an animal must expend to purposefully generate heat, and the less likely that heat generated in any metabolic pathway is useless waste.
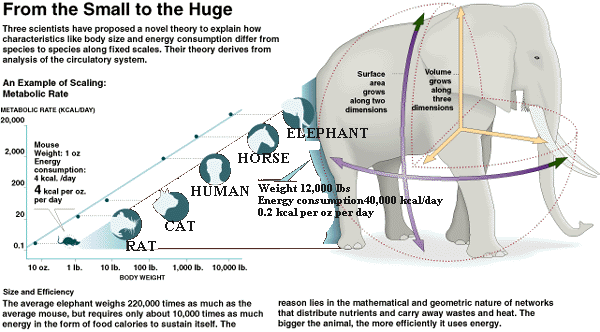
In humans, roughly half of our basal metabolic rate is for the purposes of maintaining body temperature. The heat "wastes" of our metabolic processes to "produce" energy for our body do not combine to meet this requirement and we thus generate heat as required to maintain life by other processes as well. In rodents and other small animals, this is accomplished via brown adipose tissue and a process called uncoupling in the mitochondria. This uncoupling is essentially shorting out the ATP production to produce heat instead from the membrane voltage produced in the electron transport chain (ETC). There is some indication that this mechanism can be utilized in these animals to deal with short term energy excesses as well. Adult humans have been found to have more brown fat than previously thought, but it is still negligible when compared to small animals. I can't put my finger on the citation at the moment, but studies have shown that any uncoupling in brown fat in humans is in response to cold, and goes back to normal once the cold exposure is ceased. As one would predict, because the purpose is to maintain a constant body temperature after all.
The major uncoupling protein in human skeletal muscle mitochondria is almost certainly not similar to UCP-1 in brown fat. Yes, this WILL be a subject for an upcoming post in the near future. Even if it were, there is essentially no evidence for any desirability for uncoupling of ATP synthesis for the fun of it. It seems our bodies generate "background heat" through things like "futile cycles" and from the "work" done by ATP. Unlike many other animals, we tend to require external means of temperature control such as clothing and housing at less extreme temperatures.
Efficiency ...
The bottom line here is that the term efficiency is somewhat misapplied to the human (or that of any warm blooded animal for that matter) engine. In generic terms, efficiency can be expressed as a ratio:
Many of us, particularly in the context of engine-context thermo, are conditioned to consider heat to be waste ... not something of value. In the human body, only excess heat produced would be waste. In the sense of using energy to do everything else, the concept of efficiency certainly applies, but in the context of warm blooded life, heat is rather rarely not valuable.
After all, we often produce heat for heat's sake!
* As an example of using Haynie's definitions, etc., I would cite his use of irreversible. When most hear that term we presume it to mean that the process cannot be reversed. However he uses many examples where he then goes on to discuss just that: the reverse process. Irreversible, in his contexts, means proceeding in the spontaneous direction, because in almost every example, these processes can be made to go in the reverse direction. It is just my opinion that this terminology only serves to confuse people. Even if technically correct, especially in a book with a rather informal tone throughout, it seems an odd mismatch of verbiage.


Comments
Since you never put up a photo, it would be interesting to know if you are able to apply all of your knowledge.
Thanks
Sorry, I didn't intend to offend you, I was asking, because I am curious if you are an example of all you espouse, on your blog as a whole, not one posting in particular, and does it actually work.
http://en.wikipedia.org/wiki/Equator
I could provide more, but I think you get the point. For most of the time, heat is not waste. Even if the outside temp is higher, more energy must be expended to cool the body, but core body temp is maintained "internally".
you should repeat the experiment using bacon instead, to see if there's a difference......
post when it is taken at the face value.
It is just a standard college entry-level biochemistry. However, when this post is read in the notion of human physiology/biochemistry in relation to the dietary macronutrients –
energetics reciprocation (as I presume this post was intended to) than it is ajoke; I hope it is a joke, because if it is not than it is just grotesque. Please go PubMed, or if you do not have an access to PubMed, go to your local University library – educate yourself, stop using college text-books and stop playing “I know all” in the field you are amateur. Until such time ‘do not enter the human bioenergetics room’, because you look like a deer in the head-lights in this room. It is free world we live (still) however, so you’ll do as you please including makind an ass of yourself when taking-on human bioenergetics.
Many people thing that biochemistry is easy, it is in its principals, however to grasp interconnecting concepts requires more brain power
than to comprehend Kolmogorov theorem.
Humans can actually moderately tolerate low tempratures quite easily. The real problem is that we have become accustomed to central heating and avoiding physical activities.
Of course your comment could always be a joke, because than it would be less literate then a 5th grader. Their they're, your human, me knows.
I love when someone swallows the hook and elaborates on its taste. (especially when this someone is biochemist with PhD - I do not think your husband attended my classes).
Back to the topic: are you familiar with cravings of pregnant women for foods with different tastes StellaBarbone ?. I am sure that for you and your biochemist husband, PhD, this holds no relation to this post.
(2) Tolerance to cold is through BAT thermogenesis, and all the "waste" heat from normal metabolism would reduce the amount of this required.
Note that TEF is typically 10% of total TDEE even a super thermogenic diet is unlikely to even double that which leaves us way short of the approx 50% REE that constitutes temp regulation. This "shortfall" is contributed to by "waste" heat from other energy expenditures.
Yes I do this, I know. English is not my mother tongue, it is one of several languages I attempt to communicate, which must be an annoyance for, say, Americans who are known for their eloquence and overall refinement.
Post a Comment
Comment Moderation is ON ... I will NOT be routinely reviewing or publishing comments at this time..