Insulin Resistance III: Glucose Uptake and GLUT4's
So far with this series, I've discussed the many difficulties involved in discussing and characterizing insulin resistance, IR. See Part I: A condition in dire need of diagnostic clarity and Part II: The Complexity of Hormone Resistance Phenomena. Insulin-mediated glucose transport, or "disposal" from circulation is the major action/phenomenon usually assessed or being discussed when a person (or lab animal) is described as "insulin resistant".
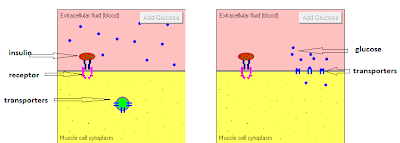
So in this part, I wanted to discuss glucose transport. This link is a nice tutorial about the action of insulin that includes an animation of ONE of its functions: glucose transport. Glucose cannot traverse membranes unassisted. In essence it must pass through protein "channels" from one side to the other. There are always glucose transporters, called GLUT's, present in the cell membrane, but they are not always at the exterior surface of the cell to receive glucose. Insulin binds to the receptor which signals movement of the transporters to the surface (translocation) allowing glucose to flow down the concentration gradient through the GLUT "channel". I've labeled the components in the screenshot below, and "taped" a short video of the animation from the tutorial so you don't have to go off-blog if you don't want to:
The GLUT4 transporters are the major insulin-stimulated glucose transporters. There are GLUT4's in muscle, adipose, and other tissues. It is important to realize that glucose transport CAN and DOES occur in the absence of insulin-stimulation of GLUT's. From this (classic!) paper:
... Most people think that hyperglycaemia reflects a reduction in glucose uptake as a result of insulin deficiency. This is not true...
... the fasting hyperglycaemia of diabetes results from hepatic over‐production of glucose alone, since peripheral glucose utilization is increased despite the lack of insulin. Insulin treatment reduces glucose concentration through inhibiting hepatic glucose production. Under these conditions glucose utilization decreases, thus insulin administration reduces glucose utilization. This indicates that the plasma glucose concentration, rather than plasma insulin, is the prime determinant of glucose uptake. There are clearly sufficient glucose transporters present, even in the newly diagnosed diabetic state, to ensure adequate glucose metabolism. Thus insulin regulates glucose production more than glucose utilization. ...
Thanks in large part to the promotion of Neel's hypotheses in GCBC, many who have learned their metabolism/physiology from Gary Taubes are under the misperception that adipose tissue is a major "sink" or tissue for glucose disposal in the postprandial state. However in rats, glucose uptake/disposal into muscle cells is 10-fold higher than that into adipose tissue. I've seen similar values for humans, and to say that muscle tissue is the major "organ" for glucose disposal in the postprandial state is pretty universally investigated, documented and accepted.
Over the past month or so, I've read here and in blog posts and comments elsewhere, many many times, how insulin resistance causes glucose to be diverted from the muscle cells to the adipose tissue to make us fat. It was/is Taubes' theory, it's Lustig's mangulation of metabolism, it's Fat Head's "you're as fat as you need to be" Fiasco, and it's what is parroted again and again. But is it true? Well, seems not. I was looking for something yesterday and a line in one of the articles I read jumped off the page at me. I've bolded it in context here:
A major cause of type 2 diabetes is impaired insulin action in adipose issue, skeletal muscle and liver. Overt hyperglycaemia develops when increased insulin secretion no longer compensates for insulin resistance. Even without diabetes, insulin resistance is a major risk factor for cardiovascular disease and early mortality. Resistance to insulin stimulated glucose transport in adipose tissue and skeletal muscle is one of the earliest defects detected in insulin-resistant states. Transmembrane transport of glucose by GLUT4 is the rate-limiting step for glucose use by muscle and adipose tissue. With the development of insulin resistance, GLUT4 expression is downregulated selectively in adipose tissue but not in skeletal muscle. Downregulation of GLUT4 expression in adipose tissue is an almost universal feature of insulin-resistant states, including obesity, type 2 diabetes and the metabolic syndrome.
Is this new? Controversial? Post-dating GCBC? Nope. My citation is from 2005, and it references articles from the late 90's. I've seen this turn up in textbook citations on Google Books. And yet, here's what GCBC says:
It is important also to know that the fat cells of adipose tissue are “exquisitely sensitive” to insulin, far more so than other tissues in the body. This means that even low levels of insulin, far below those considered the clinical symptom of hyperinsulinemia (chronically high levels of insulin), will shut down flow of fatty acids from the fat cells. Elevating insulin even slightly will increase the accumulation of fat in the cells. The longer insulin remains elevated, the longer the fat cells will accumulate fat, and the longer they’ll go without releasing it. Moreover, fat cells remain sensitive to insulin long after muscle cells become resistant to it. Once muscle cells become resistant to the insulin in the bloodstream, as Yalow and Berson explained, the fat cells have to remain sensitive to provide a place to store blood sugar, which would otherwise either accumulate to toxic levels or overflow into the urine and be lost to the body. As insulin levels rise, the storage of fat in the fat cells continues, long after the muscles become resistant to taking up any more glucose. Nonetheless, the pancreas may compensate for this insulin resistance, if it can, by secreting still more insulin. This will further elevate the level of insulin in the circulation and serve to increase further the storage of fat in the fat cells and the synthesis of carbohydrates from fat. It will suppress the release of fat from the fat tissue.
Well, this was the state of the science circa the early 1980's. It "sounds" reasonable ... makes for an "interesting argument" and all that. But is it true? Seems not. Scan back up to the red highlight above. This notion that the muscle turns glucose away and it has no place to go but adipose tissue is incorrect.
It appears that the transporters are more important than the insulin. Yes, that's right. A summary of three paragraphs from this paper (a paper I'm sure you'll be hearing more about). Italics are direct quotes, bullet points are my summary
GLUT4 Is a Key Determinant of Glucose Homeostasis
A central role for GLUT4 in whole-body metabolism is strongly supported by a variety of genetically engineered mouse models where expression of the transporter is either enhanced or ablated in muscle or adipose tissue or both.
- The whole-body GLUT4−/− mice upregulate other pathways for survival
- Partial knockout in muscle and adipose tissue result in IR and propensity towards diabetes, therefore GLUT4 plays a major role in glucose disposal
- Transgenic overexpression of GLUT4 in partial knockouts restores insulin sensitivity and glucose tolerance if GLUT4 overexpressed in muscle, transgenic mice overexpressing GLUT4 in muscle or fat tissue are highly insulin sensitive and glucose tolerant.
- Deficiency of GLUT4 in either muscle or fat produces IR and a similar propensity towards diabetes. "This was particularly surprising in the former case since adipose tissue accounts for only a small fraction of total body glucose disposal."
- Adipose GLUT4 deficiency causes IR in muscle and liver. Muscle GLUT4 deficiency causes IR in fat and liver and elevated blood glucose that can be reversed by genetically overexpressing GLUT4 in adipose tissue of these animals.
Even though cell surface GLUT4 is highly dependent on insulin in vitro, muscle-specific (MIRKO) or adipose-specific (FIRKO) insulin receptor knockout mice produce surprisingly mild metabolic phenotypes compared to the conditional GLUT4 knockout mice described above. MIRKO mice have an enlarged fat mass with increased serum triglyceride and free fatty acids, but otherwise have normal whole-body glucose homeostasis. Insulin-stimulated glucose uptake is greatly reduced in MIRKO muscle, but muscle glycogen level is normal, indicating possible compensatory mechanisms for glucose import. FIRKO mice have severe insulin resistance in adipose tissue but are protected from age- and hyperphagia-induced glucose intolerance. ... Thus, GLUT4 in muscle and adipose tissue is indispensable for normal global glucose homeostasis, while insulin receptor in these tissues appears much less critical.This work (and there's lots lots more in the cited papers, other reviews and their references) blows a massive hole through all of these blood glucose and insulin level and obesity claims. I'll end this for now by repeating a previously highlighted quote, and highlight a different part:
With the development of insulin resistance, GLUT4 expression is downregulated selectively in adipose tissue but not in skeletal muscle.
DOWNREGULATION of GLUT4 expression in adipose tissue is an almost universal feature of insulin-resistant states, including obesity, type 2 diabetes and the metabolic syndrome.
Thanks for the NEJM paper!
I didn't find an active online link to the DeFronzo paper: DeFronzo, R. A. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Rev. 5, 171–-269 (1997) If anyone has any better luck, I'd appreciate a copy. Email: carbsane at gmail dot com
I didn't find an active online link to the DeFronzo paper: DeFronzo, R. A. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Rev. 5, 171–-269 (1997) If anyone has any better luck, I'd appreciate a copy. Email: carbsane at gmail dot com
Comments
I love your blog and writing style, but I just wanted to give a quick opinion on something.
Be careful with things like this:
"However in rats, glucose uptake/disposal into muscle cells is 10-fold higher than that into adipose tissue. I've seen similar values for humans"
Whilst you provided a pubmed link for the rats, you did not provide one showing the same is true for humans. You don't want to give other readers/bloggers ammo to call you a hypocrite for using rat physiology when you may have criticised others for doing so!
http://ajpendo.physiology.org/content/289/4/E551.full
ADIPOSE-GLUT4 OVEREXPRESSION REVERSES INSULIN RESISTANCE
"...we bred muscle GLUT4 knockout (MG4KO) mice to mice over-expressing GLUT4 in adipose tissue (AG4Tg)."
"Here we find that overexpression of GLUT4 in adipose tissue not only rescues the insulin resistance resulting from impaired glucose transport in muscle but also lowers fasting glucose and glucose excursion below control levels, enhancing glucose tolerance so that it is better than in control mice. This insulin sensitivity occurs, surprisingly, in the presence of modestly increased fat mass and serum FFA concentrations and decreased serum adiponectin levels in mice overexpressing GLUT4 in adipose tissue."
"...These results reinforce the central role for adipose tissue in regulating insulin sensitivity and energy balance. The potential relevance of this model to humans is that expression of GLUT4 is selectively reduced in adipocytes of obese and type 2 diabetic individuals "
Very much looking forward to future installments.
-------------
http://www.sciencedirect.com/science/article/pii/S0024320502019483
"Compared to age-matched C mice, the 2-mo and 4-mo MSG mice were already obese, but metabolically they showed increased or preserved whole-body insulin sensitivity, respectively. At these ages they showed unchanged total GLUT4 content in SM and H. However, in plasma membrane fraction from WAT, the MSG showed increased GLUT4 content at both 2- (by 60%) and 4-month (by 45%) of age."
"...At 7 months of age, obesity was fully established in MSG mice, showing a strongly insulin resistant condition. Additionally, in the 7-mo MSG-mice the GLUT4 protein was reduced in SM (by 40%), H (by 28%), PM and M fractions of WAT (by ∼70%),"
"...In conclusion, we have demonstrated that insulin resistance of 7-month old obese MSG-treated mice involves a reduced GLUT4 protein content in skeletal muscle, heart and white adipose tissue. However, earlier, the GLUT4 content was increased in white adipose tissue without changes in muscles, and that may play an important role in the development of obesity, and later on of insulin resistance."
Thanks for the welcome on my last comment. Too little time has elapsed since I've woken for me to be witty, but I just have to point out that this statement:
"the fasting hyperglycaemia of diabetes results from hepatic over‐production of glucose alone, since peripheral glucose utilization is increased despite the lack of insulin."
is absolutely mind-boggling if one gets his information from the 'low carb researchers.'
Anyway, I think I just realized something . . . Type 2 diabetes begins in the liver, no? And then poor post-prandial glucose disposal is just a downstream result that occurs due to improper insulin signaling of GLUT4. If that's correct and I'm not just making this up as I go, what are the steps in between hepatic screwiness and systemic screwiness?
This issue has been complicated by the fact that we can dietarily induce what appears to be isolated liver IR with such things as caloric excess and either a high fat or high fructose (liquid) diet. I don't say this as absolute, and I try to park my biases at the door when I come across new studies.
What I've learned in the past month or so goes against everything low carbers tell us, or rather what they lead us to believe is undesirable in metabolism: Systemic IR/T2 is associated with reduced glyceroneogenesis and re-esterification in the internal TAG/FA cycle, impaired de novo lipogenesis in adipose tissue producing palmitoleic acid that appears to be a signaling molecule "secreted" by adipose tissue, and impaired glucose uptake in adipose tissue. All of this is consistent with a general idea of some adiposity threshold where overloaded adipocytes get "sick". Therapies that favor adipocyte differentiation ameliorate diabetes, though, unfortunately favor retention of and/or increased adiposity.
One thing for sure, fat tissue is way more complicated than one hormone!
Glucocorticoid drugs like prednisone increase gluconeogenesis (as well as increasing IR). I'm surprised that the examples of people taking those drugs for immune suppression (such as in ITP, Immune Thrombocytopenia Purpura) aren't used in all of these types of discussions.
I do remember, however, one of the supposed experts at CrossFit claiming that anyone on long term heavy doses of prednisone "will gain a lot of weight, regardless of how many calories they eat". That's typical LC nonsense of course - wherein people supposedly make fat out of thin air. However, a hallmark of long term pred use is the "moon face", which goes to redistribution and in a way that's not typical of just ordinary fat gain.
As in NEJM: "In conclusion, the administration of 1 g of n–3 fatty acids did not reduce the rate of death from cardiovascular causes or other outcomes during a period of 6 years in patients with dysglycemia and additional cardiovascular risk factors."
http://www.nejm.org/doi/full/10.1056/NEJMoa1203859#t=articleBackground
Post a Comment
Comment Moderation is ON ... I will NOT be routinely reviewing or publishing comments at this time..