Insulin Wars IV.2a: The Discussion with Todd Becker/Getting Stronger continues
If you haven't read the discussion thus far, or just to recap, here are the links:
Insulin Wars IV: Todd Becker of Getting Stronger blogInsulin Wars IV.1: Todd Becker of Getting Stronger blog responds
More Todd Becker (Getting Stronger blog) on Insulin (this last one links to his piece on his blog)
{Todd quoted the relevant prior exchanges in his email, so most can probably follow along just with this post}
I've been putting off posting this for too long, mostly because in its entirety it is very lengthy (15 pages!) and I haven't had the length of uninterrupted time to devote to a thorough point by point response to the entire email. So I decided to break this installment (IV.2) up into parts (a,b..., however many) so we can keep this discussion alive and continuing. I'm also likely to address some topics out of order from Todd's email response. Eventually it will all get up here :-) I very much appreciate all the hard work Todd put into his response and our continuing discussion. Please do bear with us, as we'll no doubt get a bit repetitive at times. I do hope you'll enjoy the complete exchange. I appreciate any heads-up on dropped links etc. that may have occurred with my C&P. Thanks again to Todd Becker!
CS: An ongoing issue I have is that you extrapolate acute (immediate) effects of insulin with chronic (long term) effects, and/or fail to include other antagonistic factors mediating the opposite effect. The association of insulin levels with degrees of fatness does not indicate causation. Most of my research shows the causation arrow points the other way - fat accumulation --> IR of fat cells --> reduced inhibition of HSL by insulin --> NEFA/FFA release --> pancreas stimulated to make more insulin.
TB: I maintain that insulin plays an important long-range causal role in fat accumulation, and not just an association or short-range cause. You propose a particular linear causal sequence above, but I’ve seen papers supporting alternate causal sequences and even causal circularity. For example, Lustig marshals extensive evidence to support his conclusion that “Although hyperinsulinemia is usually thought to be secondary to obesity, it can instead by primary, due to autonomic dysfunction.” He offers evidence that insulin acts as a leptin agonist, and that inhibiting insulin with octoreotide reversed insulin resistance, leading to spontaneous reduction in caloric intake, increased activity and weight loss. He also cites other evidence suggesting hyperinsulinemia induces leptin resistance and obesity.
CS Response: OK, the emphasis in the Lustig statement above is mine. The cited paper discusses hypothalmic obesity in children. If one does a Google search on hypothalmic obesity, they'll get a ton of hits dealing with pediatric obesity or obesity in kids after undergoing treatment for brain tumors that on rare occasions damages the hypothalmus. One such paper describes HO this way: "Hypothalamic obesity, a syndrome of intractable weight gain due to hypothalamic damage, is an uncommon but devastating complication for children surviving brain tumors.". So we're talking about a rare occurrence in a relatively rare subset of the population of children. I don't think he's saying that the obesity epidemic is due to an epidemic of spontaneous hypothalmic lesions, so I'm not really sure the relevance. I've never said that insulin can't cause fat accumulation, just that insulin doesn't just "go wild" and do all of the things it is capable of in a chronic fashion from dietary stimuli.
I'm not as well versed in leptin as I'd like to be, but whatever the malfunction of the hypothalmus, this is causing the pancreas' of these kids and others so afflicted, to put out a ton of insulin. But as Lustig himself states, this is not how hyperinsulinemia is thought to develop [in the majority of cases]. What causes hyperinsulinemia in the rest of the population? I have read many attributing it to leptin resistance in the brain/hypothalmus, and I open the floor to my readers to link me to any and all information you have on dietary instigators of this as a chronic condition. These HO kids have a chronic problem due to "mechanically" damaged brains. For that to be relevant in others we would have to identify other ways to induce chronic malfunctions of similar pathways.
Further supporting this conclusion is a study by Woodhouse et al. showing that insulin reduction actually precedes fat loss in exercising individuals, suggesting that elevated insulin is a cause of obesity.
I'm going to quote the Woodhouse abstract below (don't have full text access) and address this and your interpretation.
The present study examined fasting serum insulin levels in relation to body composition and dietary intake during the initial 4 weeks of a 12-week physical training programme in 26 previously sedentary men. Fasting serum insulin concentrations decreased markedly during the first 4 weeks of training and remained at these reduced levels for the rest of the study. The early fall in serum insulin concentration was significantly correlated with the concomitant decrease in body fat, the increase in lean body weight and the age of the subjects. Body weight and reported dietary intake on the other hand, did not change significantly over this period. These results indicate that the decrease in fasting serum insulin in previously sedentary men with physical training is associated with the concomitant changes in body composition. Increased muscle tissue in particular may contribute to this training-induced decrease in serum insulin.
Without the full study here, I'm not sure what to make of this one other than to say that it looks like fatty acids are moved from fat to muscle because, interestingly, there was no overall weight loss. Since insulin stimulates glycogen synthesis, and one might expect sedentary people to rely more on glycogenolysis in the early going, the reduction in insulin is not surprising. Nor is it staying lower for when more fatty acids are needed to be liberated from adipose tissue to be used for energy.
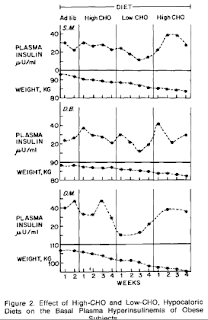
Again, I'm going to quote the abstract (transcribed from scan w/abbrev)And a study by Grey and Kipnis showed that a low carbohydrate diet reduced basal insulin by 50%, followed by weight loss, whereas refeeding an isocaloric high carbohydrate diet re-raised basal insulin (not just “insulin spikes” as James Krieger claims) even before any weight regain.
During two successive three-week periods, seven obese subjects were fed isocaloric diets, the first low, the second high in carbohydrate. In all, basal insulin levels decreased 50% on the LC diet and increased on the HC diet. Three obese subjects were fed, during three successive 4-week periods, 1500 cal diets with high, then low, then high carb content. Basal plasma insulin levels were significantly reduced on the LC diet. Refeeding of the HC diet, despite continued weight loss, resulted in markedly increased basal plasma insulin. In both protocols, most patients also exhibited a decreased insulin secretory response to oral glucose when on LC and an increased response on HC.
Thus the hyperinsulinemia characteristic of obesity may be a result, in part, of dietary factors rather than exclusively a consequence of the insulin antagonism associated with obesity.
First, I would like to address the part I emphasized, where insulin ROSE despite continued weight LOSS. Umm, if insulin causes fat accumulation, should not the carb induced rise in insulin have caused weight gain? If one looks at the figure below, weight loss remained relatively steady throughout the changes and seemingly had nothing to do with insulin levels that varied considerably.
The more I look at that above figure, I don't see how more "graphic" it could get demonstrating a lack of correlation of fat accumulation with insulin levels.
What this study showed is that in obese individuals with established hyperinsulinemia, dietary carb can contribute to the mess. This is something that I plan to address in my series on IR (if I ever get to it!), especially why it seems LC is so effective for the insulin resistant in the early going. But this provides no insight as to carbohydrates and insulin spikes per se leading to chronic elevations in basal insulin. Even if there was a correlation (I welcome data if anyone has it!) between carb intake and basal insulin, I would look to the Japanese and ask why they're not fat then.
What this study showed is that in obese individuals with established hyperinsulinemia, dietary carb can contribute to the mess. This is something that I plan to address in my series on IR (if I ever get to it!), especially why it seems LC is so effective for the insulin resistant in the early going. But this provides no insight as to carbohydrates and insulin spikes per se leading to chronic elevations in basal insulin. Even if there was a correlation (I welcome data if anyone has it!) between carb intake and basal insulin, I would look to the Japanese and ask why they're not fat then.
Below is a Figure 4 from Lustig's paper:Of course, Lustig does also acknowledge the flip side: that diet-induced obesity can in turn induce hyperinsulinemia. So perhaps a causal “web” with appropriate feedback loops is more representative of the truth, than a linear causal sequence. From a review of 80 references, Lustig pieces together a causal “web” (see his Figure 4) in which insulin resistance and hyperinsulinemia play a central role ...
... leading to obesity and leptin resistance, with feedback loops in which obesity reinforces the insulin resistance.
I'm not seeing the feedback loop where obesity reinforces insulin resistance here. Indeed the arrow seems to go one way. To me, such a web could only be constructed through cherry-picking. I would point out to everyone reading this, however, that Lustig implicates a high fat diet in the development of both IR and leptin resistance, and low activity contributing to IR and to obesity. (I'm sure someone will jump on me for mentioning this, but sounds like Lustig has a different idea of "Why We Get Fat" than Taubes). Where obesity and IR are concerned, the science I'm researching points to the majority direction arrow to be obesity leading to IR. I'll again reference: The Progression of IR, Adipose Tissue & IR, Fat Fails First?
Lustig’s web includes additional afferent factors that stimulate appetite and efferent factors that modulate feeding behavior. You may disagree with some specifics in the web, but I assume you would agree that a causal web is plausible in biology.
I certainly would agree that such web is not only plausible, but likely. But at the risk of repeating myself, Lustig seems to have missed the immense body of literature pointing arrows in the other direction in his web.
Furthermore, even if you are right that ASP can function at very low insulin levels, its activity increases with increasing insulin levels. Thus, insulin plays an important role as an enabler of ASP. Of even greater significance, however, insulin upregulates LPL and inhibits HSL. This clearly shifts the balance toward more fat accumulation and less fat mobilization. As a result, people who are hyperinsulinemic have far more difficulty than do others in mobilizing their fat stores. Reduced ability to mobilize fat or glucose tends to increase hunger, more frequent eating and further weight gain. For all these reasons, I think it is fair to consider elevated insulin as key causal contributor to obesity. To use your bath tub analogy, if I plug a tub while the spigot is running, and the water overflows, the running water is not the sole cause of the filling; the plug is also a cause, because without it the tub would not fill up and would remain empty.
Here is where I'm talking about short term v. long term action. Something James Krieger addressed in his articles. We always cycle fat in our adipose tissue. The obese, in every study I can recall where its measured and compared to lean, almost always have elevated NEFA. This indicates either inefficient trapping of FA's released by LPL action on chylo or inefficient insulin action on HSL failing to suppress FA release from adipose tissue, or, likely, both.
More than one of my readers has commented on this blog regarding the evolutionary or survival based untenability of the "insulin required" theory for fat accumulation. It is unlikely that our ancestors fired up some tuber fries to go with the animal kill whose fat we ate. It would make no sense to not have a means by which to store that fat for a period of famine.
My bottom line on all of this insulin as causal factor is that it cannot explain the obesity epidemic. Did all these Western societies develop some sort of hypothalmic dysfunction en masse circa 1980? Lustig would blame fructose I suppose, but how to explain why the sugar rats faired no worse weight-wise (slightly better but not stat.sig.) than the relatively low carb high fat chow rats in this study, blogged on here. If insulin is the controlling factor and/or intertwined with leptin, CAUSING fat accumulation, how do you explain the results in this study? The HF group ate a 45% fat (lard) chow that was relatively low carb (35%) while the LF group ate a matched chow (although 10% fat was lard + O6 soybean oil) and 35% of fat composition was replaced with sucrose (therefore IMO they should have called this an HS diet!). The sugar rats consumed 2.5X the total carbs and simple carbs in the form of sucrose compared to the high fat rats yet fat accumulated similarly in both groups. Compared to the standard chow, the high fat rats consumed half the carbs and even fewer calories, but accumulated more fat. This study flies in the face of insulin as causal factor.
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
TB: It is probably true that eating more fat makes you fatter, but from what I’ve read this process itself requires some threshold level insulin – which requires carbohydrates or – to a lesser extent – proteins. Eating high fat meals with zero or very low carbs or proteins helps promote net fat loss. I’ve experienced the benefits of a “fat fast” myself (although I don’t think it is so great as a sustainable diet by itself) as have many others. If you want I can try to find studies to document this. Have you found any evidence that eating fat without carbs or protein can increase body fat?
CS: Unless you're a type 1 diabetic, you always have some insulin around, but insulin is not needed. It may further stimulate ASP, but ASP can do its job -- as the main "hormone" regulating esterification -- just fine on its own. Several relevant blog posts HERE. But, there's an overfeeding study (don't have a link, perhaps a reader can help here?) where on top of ~150g carbs subjects were fed up to 600g fat. They didn't gain exactly the predicted weight, but they sure put on fat massH And, if insulin is the ultimate determinant of fat accumulation, how do you explain why carb overfeeding resulted in 75-85% storage of excesses, while fat overfeeding resulted in 90-95% of excesses stored.?
TB: Obviously there is always some minimal amount of insulin present, except in the case of Type 1 diabetics. But lipolysis of stored fat does not require that insulin levels are zero, merely that they are low. It’s a continuum: the lower the insulin, the greater the relative shift towards fatty acid mobilization and oxidation. And there is a significant difference between having a low basal insulin (say, less than 6 uU/ml) and a moderately elevated insulin level (more than 12 uU/ml). Reducing basal insulin promotes sustainable weight loss, whether one gets there by low carb, low fat, calorie restriction, intermittent fasting, exercise, or combinations of the above. I’m not that concerned about transient insulin spikes, so long as insulin levels are reduced sufficiently between meals in order to mobilize significant amounts of fat. For those who are hyperinsulinemic or insulin resistant, or even just eating too many carbohydrates, insulin levels may never get low enough to allow lipolysis for very much time out of each day. By using intermittent fasting in combination with moderately low carb, I lowered my basal insulin to 4 uU/ml and 35 pounds. The reduced insulin levels came before most of the weight loss.
I’d really like to see you produce some data showing that a diet high in fat and very low in carbs can lead to significant and sustained fat accumulation, to substantiate your theory that ASP is sufficient to drive fat accumulation, even at low insulin levels. Notice again that I did not say zero insulin; just low insulin (or low carb as a proxy for this). The overfeeding study you cite still had 150 carbs, so insulin would have been elevated. Your study only showed that overfeeding fat, on top of a significant (150 g) amount of carbs, led to more efficient storage of the excess calories than did overfeeding of carbs on top of the same baseline 150 g carbs. That’s not surprising: it is well known that carbs plus fat is more fattening than either alone. What I’m looking for is a study with very low carbs (20 grams or less) or protein (50 grams or less) to minimize insulin generation. With the upregulated HSL and downregulated LPL, can ASP still drive net fat accumulation?
I'm posting this with minimal comment. I do not know if a VLC overfeeding study has been done in humans, but I've blogged on rats getting fat (and diabetic) on VLC diets. Obviously SOMETHING in these rats drives fat accumulation. Anecdotally, there are many low carbers who have gained significant weight eating very low carb. Jimmy Moore had gained almost 80 lbs eating quite consistently low carb for more than two years. I've also read literally hundreds of studies that report insulin levels on weight loss and weight stable diets. In some insulin is lower, in some it's not, short vs. long term studies tend to give different results, and as I've cited repeatedly, in Shai, the diabetics had a different result than the non-diabetics comparing LC, HF and Med diets. GLP-1 (that's in Lustig's "web") is an intestinally produced incretin that, among other things, stimulates insulin production. GLP-1 is produced in the distal small intestine in response to lipids (and other nutrients). It may well be part of the way insulin magnifies ASP. Still, chylo's themselves stimulate ASP in vitro by an order of magnitude more than insulin, so I remain unconvinced that esterification requires much insulin if at all. Question: Why do Type II's often keep gaining weight? Answer: ASP, probably.
To be continued ....

Comments
Hepatic IR = Insufficient Glucose Intake & Excessive Glucose Production.
Adipose IR = Excessive NEFA/Glycerol Production.
Skeletal Muscle IR = Insufficient Glucose/NEFA Intake.
For what it's worth, and for any who care here's an overview of good selection of overfeeding studies.
http://www.nutritionandmetabolism.com/content/3/1/25#B10
Nice find!
I forgot to mention that in the study Matt Stone has linked to a couple of times: http://www.ajcn.org/content/62/1/19.full.pdf+html that regardless of baseline carb intake, adding more carbs (insulin) vs. fat contributed to greater fat gains for the fat intake v. matched carb intake.
I also have some that I didn't find in that review:
[1] Whitley H.A., et al. Metabolic responses to isoenergetic meals containing different proportions of carbohydrate and fat. http://pmid.us/9292756
[2] Abbott W.G., et al. Short-term energy balance: relationship with protein, carbohydrate, and fat balances. Am J Physiol. 1988 Sep;255(3 Pt 1):E332-7. http://pmid.us/3421330
Quote: "Thus fat, rather than carbohydrate or protein, is almost exclusively used or stored in response to day-to-day fluctuations in energy balance".
[3] Bennett C., et al. Short-term effects of dietary-fat ingestion on energy expenditure and nutrient balance. Am J Clin Nutr. 1992 Jun;55(6):1071-7. http://pmid.us/1595577
Quote: "Acutely, dietary fat ingested in excess of its usual rate of oxidation appears to be stored in the body. Being physically fit does not appear to provide an advantage in avoiding short-term storage of excess dietary fat".
[4] Schutz Y., et al. Failure of dietary fat intake to promote fat oxidation: a factor favoring the development of obesity. Am J Clin Nutr. 1989 Aug;50(2):307-14. http://pmid.us/2756918
Quote: "These data indicate that substantial imbalances between intake and oxidation are much more likely for fat than for carbohydrate".
I haven't read them yet in detail (continually growing to-read list...).
Fat in excess seems fattening, at least in the short-term. In the longer term I would guess that leptin goes up, causing higher metabolism and lower apetite. But I still need to learn a lot about leptin.
John
1) Carbs & fat were approximately equally fattening in this study.
2) More body fat at baseline -> gained more weight on high carb
3) Less body fat at baseline -> more carb-tolerant
Could points 2 and 3 be combined to say that carbs won't make lean people fat, but will make fat people fatter? in this scenario, obesity might be a pre-requisite for carb-intolerance (?)
@John: A researcher after my own heart! I love reviews like that paper as they tend to provide a goldmine of references. My reading list is soooo backed up! Also wish they had these online sites like PubMed that list related articles or articles that cite this or that back when I was in grad school.
Post a Comment
Comment Moderation is ON ... I will NOT be routinely reviewing or publishing comments at this time..